Phosphorodithioates on:
[Wikipedia]
[Google]
[Amazon]
Thiophosphates (or phosphorothioates, PS) are
 The simplest thiophosphates have the formula S4−''x''O''x''sup>3−. These trianions are only observed at very high pH, instead they exist in protonated form with the formula ''n''PS4−''x''O''x''sup>(3−''n'')− (x = 0, 1, 2, or 3 and (n = 1, 2, or 3).
The simplest thiophosphates have the formula S4−''x''O''x''sup>3−. These trianions are only observed at very high pH, instead they exist in protonated form with the formula ''n''PS4−''x''O''x''sup>(3−''n'')− (x = 0, 1, 2, or 3 and (n = 1, 2, or 3).
 Monothiophosphate is the anion O3Ssup>3−, which has C3v
Monothiophosphate is the anion O3Ssup>3−, which has C3v
 A number of these anions known. Some have attracted interest as components in fast ion conductors for use in solid state batteries. The binary thiophosphates do not exhibit the extensive diversity of the analogous oxyanions but contain similar structural features, for example P is 4 coordinate, P−S−P links form and there are P−P bonds. One difference is that ions may include polysulfide fragments of 2 or more S atoms whereas in the P−O anions there is only the reactive −O−O−, peroxo, unit.
* is the analogue of the
A number of these anions known. Some have attracted interest as components in fast ion conductors for use in solid state batteries. The binary thiophosphates do not exhibit the extensive diversity of the analogous oxyanions but contain similar structural features, for example P is 4 coordinate, P−S−P links form and there are P−P bonds. One difference is that ions may include polysulfide fragments of 2 or more S atoms whereas in the P−O anions there is only the reactive −O−O−, peroxo, unit.
* is the analogue of the
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s and anions with the general chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
(''x'' = 0, 1, 2, or 3) and related derivatives where organic groups are attached to one or more O or S. Thiophosphates feature tetrahedral phosphorus(V) centers.
Organic
Organothiophosphates are a subclass oforganophosphorus compounds
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective ins ...
that are structurally related to the inorganic thiophosphates. Common members have formulas of the type (RO)3−''x''R''x''PS and related compounds where RO is replaced by RS. Many of these compounds are used as insecticides, some have medical applications, and some have been used as oil additive
Oil additives are chemical compounds that improve the lubricant performance of base oil (or oil "base stock"). The manufacturer of many different oils can utilize the same base stock for each formulation and can choose different additives for each ...
s.J. Svara, N. Weferling, T. Hofmann "Phosphorus Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006.
Oligonucleotide phosphorothioates (OPS) are modified oligonucleotides where one of the oxygen atoms in the phosphate moiety is replaced by sulfur. They are the basis of antisense therapy, e.g., the drugs fomivirsen
Fomivirsen (brand name Vitravene) is an antisense antiviral drug that was used in the treatment of cytomegalovirus retinitis (CMV) in immunocompromised patients, including those with AIDS. It was administered via intraocular injection.
It was d ...
(Vitravene), oblimersen
Oblimersen (INN, trade name Genasense; also known as Augmerosen and bcl-2 antisense oligodeoxynucleotide G3139) is an antisense oligodeoxyribonucleotide being studied as a possible treatment for several types of cancer, including chronic lymphocy ...
, alicaforsen
Alicaforsen (trade name Camligo) is an antisense oligonucleotide therapeutic that targets the messenger RNA for the production of human ICAM-1 receptor and is being developed for the treatment of acute disease flares in moderate to severe Inflamm ...
, and mipomersen
Mipomersen (INN; trade name Kynamro) is a drug used to treat homozygous familial hypercholesterolemia and is administered by subcutaneous injection. There is a serious risk of liver damage from this drug and it can only be prescribed in the cont ...
(Kynamro).
Inorganic
 The simplest thiophosphates have the formula S4−''x''O''x''sup>3−. These trianions are only observed at very high pH, instead they exist in protonated form with the formula ''n''PS4−''x''O''x''sup>(3−''n'')− (x = 0, 1, 2, or 3 and (n = 1, 2, or 3).
The simplest thiophosphates have the formula S4−''x''O''x''sup>3−. These trianions are only observed at very high pH, instead they exist in protonated form with the formula ''n''PS4−''x''O''x''sup>(3−''n'')− (x = 0, 1, 2, or 3 and (n = 1, 2, or 3).
Monothiophosphate
 Monothiophosphate is the anion O3Ssup>3−, which has C3v
Monothiophosphate is the anion O3Ssup>3−, which has C3v symmetry
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definit ...
. A common salt is sodium monothiophosphate
Sodium monothiophosphate, or sodium phosphorothioate, is an inorganic compound with the molecular formula Na3PO3S(H2O)x. All are white solids. The anhydrous material (x = 0) decomposes without melting at 120-125 °C. More common is the dode ...
(Na3PO3S). Monothiophosphate is used in research as an analogue of phosphate in biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
. Monothiophosphate esters are biochemical reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s used in the study of transcription
Transcription refers to the process of converting sounds (voice, music etc.) into letters or musical notes, or producing a copy of something in another medium, including:
Genetics
* Transcription (biology), the copying of DNA into RNA, the fir ...
, substitution interference assays. Sometimes, "monothiophosphate" refers to esters such as (CH3O)2POS−.
Dithiophosphates
Dithiophosphate has the formula O2S2sup>3−, which has C2vsymmetry
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definit ...
. Sodium dithiophosphate
Sodium dithiophosphate is the salt with the formula Na3PS2O2. It is usually supplied as the hydrated solid or as an aqueous solution together with other thiophosphates such as sodium monothiophosphate and sodium trithiophosphate. It is a colorl ...
, which is colorless, is the major product from the reaction of phosphorus pentasulfide with NaOH:R. Klement "Phosphorus" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1., p. 571.
:P2S5 + 6 NaOH → 2 Na3PO2S2 + H2S + 2 H2O
Dithiophosphoric acid is obtained by treatment of barium dithiophosphate with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
:
:Ba3(PO2S2)2 + 3 H2SO4 → 3 BaSO4 + 2 H3PO2S2
Both Na3PO2S2 and especially H3PO2S2 are prone toward hydrolysis to their monothio derivatives.
Tri- and tetrathiophosphates
Trithiophosphate is the anion OS3sup>3−, which has C3v symmetry. Tetrathiophosphate is the anion S4sup>3−, which has Td symmetry.P''x''S''y'': binary thiophosphates and polyphosphates
 A number of these anions known. Some have attracted interest as components in fast ion conductors for use in solid state batteries. The binary thiophosphates do not exhibit the extensive diversity of the analogous oxyanions but contain similar structural features, for example P is 4 coordinate, P−S−P links form and there are P−P bonds. One difference is that ions may include polysulfide fragments of 2 or more S atoms whereas in the P−O anions there is only the reactive −O−O−, peroxo, unit.
* is the analogue of the
A number of these anions known. Some have attracted interest as components in fast ion conductors for use in solid state batteries. The binary thiophosphates do not exhibit the extensive diversity of the analogous oxyanions but contain similar structural features, for example P is 4 coordinate, P−S−P links form and there are P−P bonds. One difference is that ions may include polysulfide fragments of 2 or more S atoms whereas in the P−O anions there is only the reactive −O−O−, peroxo, unit.
* is the analogue of the nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
ion, (there is no analogue); it was isolated as the yellow tetraphenylarsonium salt
* is the sulfur analogue of , and like is tetrahedral.
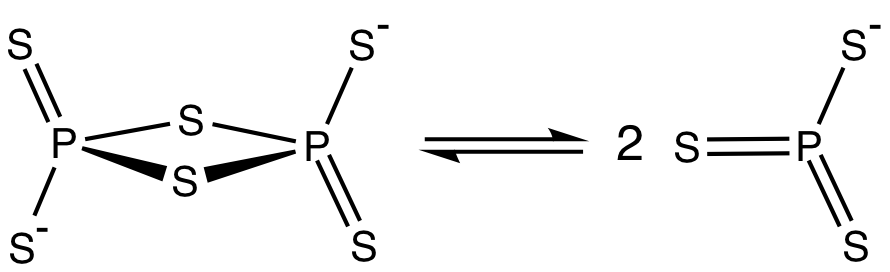
* the pyrothiophosphate ion consisting of two corner sharing PS4 tetrahedra, analogous to the pyrophosphates.Phosphorus: Chemistry, Biochemistry and Technology, Sixth Edition, 2013, D.E.C. Corbridge, CRC Pres, Taylor Francis Group,
* An ion which can be visualised either as two PS4 tetrahedra joined by a disulfide link or a pyrothiophosphate where the bridging −S− is replaced by −S4−.
* edge-shared bitetrahedral structure. The structure is therefore similar to the isoelectronic Al2Cl6 dimer. The oxygen analogue, dimetaphosphate , in contrast, is not known, the metaphosphates favour polymeric structures of chains or rings.
* and are related to but their two bridging −S− atoms are replaced by −S−S− in and by an −S−S−S− bridge in .
*P2S These form water-stable salts. The anion has an ethane-like structure and contains a P−P bond. The formal oxidation state of phosphorus is +4. The oxygen analogue is the hypodiphosphate anion, .
* contains a six-membered P3S3 ring. The ammonium salt is produced by reaction of P4S10 in liquid ammonia. Another way of visualising the structure is that it is the P4S10 adamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the m ...
(P4O10) structure with a PS3+ vertex removed.
* contains a square P4 ring, contains a P5 ring and a P6 ring. These cyclic anions contain P with an oxidation state +3. Note they are not trigonal as arsenic(III) is in arsenites, but are tetrahedral with two bonds to other phosphorus atoms and two to sulfur. The anion is analogous to the ring anion.
* An unusual butterfly-shaped ion, SP(P2)PS, which can be visualised as a P4 molecule where two P−S bonds replace one P−P bond.
* is a sulfido heptaphosphane cluster anion.
References
{{Organophosphorus Anions Sulfur ions Functional groups