Phosphaethynolate on:
[Wikipedia]
[Google]
[Amazon]
The phosphaethynolate
 Ten years later, in 2002, Westerhausen et al. published the use of Becker's method to make a family of alkaline earth metal
Ten years later, in 2002, Westerhausen et al. published the use of Becker's method to make a family of alkaline earth metal  It was not until 2011 that the first stable salt of the phosphaethynolate anion was reported by Grutzmacher and co-workers . They managed to isolate the compound as a brown solid in 28% yield. The structure of the stable sodium salt, formed by carbonylation of sodium phosphide, contains bridging PCO units in contrast to the terminal anions found in the previously reported structures. The authors noted that this sodium salt could be handled in air as well as water without major decomposition; this emphasises the significance of the accompanying counter cation in stabilisation of PCO.
It was not until 2011 that the first stable salt of the phosphaethynolate anion was reported by Grutzmacher and co-workers . They managed to isolate the compound as a brown solid in 28% yield. The structure of the stable sodium salt, formed by carbonylation of sodium phosphide, contains bridging PCO units in contrast to the terminal anions found in the previously reported structures. The authors noted that this sodium salt could be handled in air as well as water without major decomposition; this emphasises the significance of the accompanying counter cation in stabilisation of PCO.
 Direct
Direct 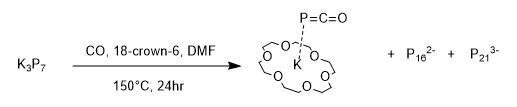
 The phosphaethynolate anion is the heavier
The phosphaethynolate anion is the heavier
 Coordination via the oxygen atom is favoured by hard, highly electropositive centres. This is due to the fact that oxygen is a more electronegative atom and thus prefers to bind via more ionic interactions. Examples of this type of coordination were presented in the work of Arnold et al. from 2015. The group found that actinide complexes of PCO involving
Coordination via the oxygen atom is favoured by hard, highly electropositive centres. This is due to the fact that oxygen is a more electronegative atom and thus prefers to bind via more ionic interactions. Examples of this type of coordination were presented in the work of Arnold et al. from 2015. The group found that actinide complexes of PCO involving




 The simplest analogue that can be formed as 'X' is varied is . This anion was first isolated by Becker et al. by reacting the phosphaethynolate anion with carbon disulphide. Unlike PCO, PCS shows ambidentate nucleophilic tendencies towards the W(0) complex mentioned above.
This is the result of a reduced difference in electronegativity between E and X thus neither atom offers a substantial advantage over the other in terms of providing ionic contributions to bonding. As a result, the average electron density in PCS is spread over the entire anion whereas in PCO, most electron density is localised on the phosphorus atom as this is the atom which bonds to form the thermodynamically favourable product.
The simplest analogue that can be formed as 'X' is varied is . This anion was first isolated by Becker et al. by reacting the phosphaethynolate anion with carbon disulphide. Unlike PCO, PCS shows ambidentate nucleophilic tendencies towards the W(0) complex mentioned above.
This is the result of a reduced difference in electronegativity between E and X thus neither atom offers a substantial advantage over the other in terms of providing ionic contributions to bonding. As a result, the average electron density in PCS is spread over the entire anion whereas in PCO, most electron density is localised on the phosphorus atom as this is the atom which bonds to form the thermodynamically favourable product.
doi:10.1039/c4qo00189c
Camp, C., Settineri, N., Lefèvre, J., Jupp, A. R., Goicoechea, J. M., Maron, L. and Arnold, J. (2015) 'Uranium and thorium complexes of the phosphaethynolate ion', ''Chemical Science''
doi: 10.1039/c5sc02150b
Heift, D., Benko, Z. and Grützmacher, H. (2014) 'Is the phosphaethynolate anion, (OCP)-, an ambident nucleophile? A spectroscopic and computational study', ''Dalton Transactions''
doi: 10.1039/c3dt53569j
Jupp, A. R., and Goicoechea, J. M. (2013) 'Phosphinecarboxamide: A Phosphorus-Containing Analogue of Urea and Stable Primary Phosphine', ''J. Am. Chem. Soc.''
DOI:10.1021/ja4115693
Becker, G., Schwarz, W., Seidler, N. and Westerhausen, M. (1992) 'Acyl‐ und Alkylidenphosphane. XXXIII. Lithoxy‐methylidenphosphan · DME und ‐methylidinphosphan · 2 DME — Synthese und Struktur', ''ZAAC ‐ Journal of Inorganic and General Chemistry''
doi: 10.1002/zaac.19926120113
Grutzmacher, H., and Goicoechea, J. (2018) 'The chemistry of the 2‐phosphaethynolate anion', ''Angew. Chem. Int. Ed''. doi
10.1002/anie.201803888
Pyykkö, P. (2015) 'Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: A summary', ''Journal of Physical Chemistry A''
doi: 10.1021/jp5065819
Westerhausen, M., Schneiderbauer, S., Piotrowski, H., Suter, M. and Nöth, H. (2002) 'Synthesis of alkaline earth metal bis(2-phosphaethynolates)', ''Journal of Organometalic Chemistry''
doi: 10.1016/S0022-328X(01)01267-0
Puschmann, F. F., Stein, D., Heift, D., Hendriksen, C., Gal, Z. A., Grützmacher, H. F. and Grützmacher, H. (2011) 'Phosphination of carbon monoxide: A simple synthesis of sodium phosphaethynolate (NaOCP)', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201102930
Jupp, A. R. and Goicoechea, J. M. (2013) 'The 2-phosphaethynolate anion: A convenient synthesis and +2cycloaddition chemistry', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201305235
Hou, G. L., Chen, B., Transue, W. J., Yang, Z., Grützmacher, H., Driess, M., Cummins, C. C., Borden, W. T. and Wang, X. Bin (2017) 'Spectroscopic Characterization, Computational Investigation, and Comparisons of ECX-(E = As, P, and N; X = S and O) Anions', ''Journal of the American Chemical Society''
doi: 10.1021/jacs.7b02984
Alidori, S., Heift, D., Santiso-Quinones, G., Benkå, Z., Grützmacher, H., Caporali, M., Gonsalvi, L., Rossin, A. and Peruzzini, M. (2012) 'Synthesis and characterization of terminal e(XCO)(CO) 2(triphos)(X=N, P): Isocyanate versus phosphaethynolate complexes', ''Chemistry - A European Journal''
doi: 10.1002/chem.201202590
Jupp, A. R., Geeson, M. B., McGrady, J. E. and Goicoechea, J. M. (2016) 'Ambient-Temperature Synthesis of 2-Phosphathioethynolate, PCS-, and the Ligand Properties of ECX-(E = N, P; X = O, S)', ''European Journal of Inorganic Chemistry''
doi: 10.1002/ejic.201501075
Robinson, T. P., Cowley, M. J., Scheschkewitz, D. and Goicoechea, J. M. (2015) 'Phosphide delivery to a cyclotrisilene', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201409908
Tambornino, F., Hinz, A., Köppe, R. and Goicoechea, J. M. (2018) 'A General Synthesis of Phosphorus- and Arsenic-Containing Analogues of the Thio- and Seleno-cyanate Anions', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201805348
Lu, Y., Wang, H., Xie, Y., Liu, H. and Schaefer, H. F. (2014) 'The cyanate and 2-phosphaethynolate anion congeners ECO-(E = N, P, As, Sb, Bi): Prelude to experimental characterization', ''Inorganic Chemistry''
doi: 10.1021/ic500780h
Szkop, K., Jupp, A. R., and Stephan, D. W. (2018) 'P,P‑Dimethylformylphosphine: The Phosphorus Analogue of N,N‑Dimethylformamide' ''J. Am. Chem. Soc''
doi:10.1021/jacs.8b09266
Chen, X., Alidori, S., Puschmann, F. F., Santiso-Quinones, G., Benko, Z., Li, Z., Becker, G., Grützmacher, H. F. and Grützmacher, H. (2014) 'Sodium phosphaethynolate as a building block for heterocycles', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201308220
Hinz, A., Labbow, R., Rennick, C., Schulz, A. and Goicoechea, J. M. (2017) 'HPCO—A Phosphorus-Containing Analogue of Isocyanic Acid', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201700368
Tondreau, A. M., Benko, Z., Harmer, J. R. and Grützmacher, H. (2014) 'Sodium phosphaethynolate, Na(OCP), as a “p” transfer reagent for the synthesis of N-heterocyclic carbene supported P3 and PAsP radicals', ''Chemical Science''
doi: 10.1039/c3sc53140f
Anions Organophosphorus compounds Physical organic chemistry Substances discovered in the 1990s
anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
, also referred to as PCO, is the phosphorus-containing analogue of the cyanate
Cyanate is an anion with the structural formula , usually written . It also refers to any salt containing it, such as ammonium cyanate.
It is an isomer of the much less stable fulminate anion .William R. Martin and David W. Ball (2019): "Sm ...
anion with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
or . The anion has a linear geometry and is commonly isolated as a salt. When used as a ligand, the phosphaethynolate anion is ambidentate in nature meaning it forms complexes by coordinating via either the phosphorus or oxygen atoms. This versatile character of the anion has allowed it to be incorporated into many transition metal and actinide complexes but now the focus of the research around phosphaethynolate has turned to utilising the anion as a synthetic building block to organophosphanes.
Synthesis
The first reportedsynthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
and characterisation of phosphaethynolate came from Becker et al. in 1992. They were able to isolate the anion as a lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
salt (in 87% yield) by reacting lithium bis(trimethylsilyl)phosphide with dimethyl carbonate . The x-ray crystallographic analysis of the anion determined the bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest ...
to be (indicative of a phosphorus-carbon triple bond) and the bond length to be . Similar studies were performed on derivatives of this structure and the results indicated that dimerisation to form a four-membered Li ring is favoured by this molecule.
 Ten years later, in 2002, Westerhausen et al. published the use of Becker's method to make a family of alkaline earth metal
Ten years later, in 2002, Westerhausen et al. published the use of Becker's method to make a family of alkaline earth metal salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively ...
of PCO ; this work involved the synthesis of the magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar t ...
, strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ...
and barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
...
bis-phosphaethynolates. Like the salts previously reported by Becker, the alkali-earth metal analogues were unstable to moisture and air and thus were required to be stored at low temperatures (around ) in dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is misc ...
solutions.
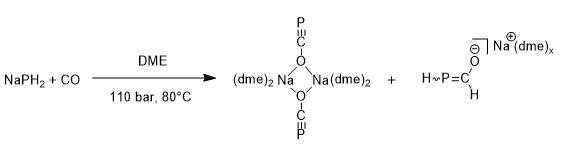
 It was not until 2011 that the first stable salt of the phosphaethynolate anion was reported by Grutzmacher and co-workers . They managed to isolate the compound as a brown solid in 28% yield. The structure of the stable sodium salt, formed by carbonylation of sodium phosphide, contains bridging PCO units in contrast to the terminal anions found in the previously reported structures. The authors noted that this sodium salt could be handled in air as well as water without major decomposition; this emphasises the significance of the accompanying counter cation in stabilisation of PCO.
It was not until 2011 that the first stable salt of the phosphaethynolate anion was reported by Grutzmacher and co-workers . They managed to isolate the compound as a brown solid in 28% yield. The structure of the stable sodium salt, formed by carbonylation of sodium phosphide, contains bridging PCO units in contrast to the terminal anions found in the previously reported structures. The authors noted that this sodium salt could be handled in air as well as water without major decomposition; this emphasises the significance of the accompanying counter cation in stabilisation of PCO.
 Direct
Direct carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbo ...
was a method also employed by Goicoechea in 2013 in order to synthesis a phosphaethynolate anion stabilised by a potassium cation sequestered in 18-crown-6 . This method required the carbonylation of solutions of at and produced by-products that were readily separated during aqueous work ups. The use of aqueous work ups reflects the high stability of the salt in water. This method afforded the PCO anion in reasonable yields around 43%. Characterisation of the compound involved infra-red spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or funct ...
; the band indicative of the triple bond stretch was observed at .

Ambidentate nature of the anion
 The phosphaethynolate anion is the heavier
The phosphaethynolate anion is the heavier isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
congener of the cyanate anion. It has been shown that it behaves in a similar way to its lighter analogue, as an ambidentate nucleophile. This ambidentate character of the anion means that it is able to bind via both the phosphorus and oxygen atoms depending on the nature of the centre being coordinated.
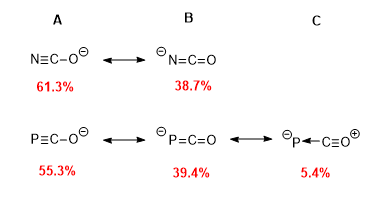
Computational studies carried out on the anion such as Natural Bond Orbital (NBO) and Natural Resonance Theory (NRT) analyses can go part way to explain why PCO can react in such a manner . The two dominant resonance forms of the phosphaethynolate anion localise negative charge on either the phosphorus or oxygen atoms meaning both are sites of nucleophilicity. The same applies for the cyanate anion hence why PCO is observed to have similar pseudo-halogenic behaviour.
Attack by oxygen
 Coordination via the oxygen atom is favoured by hard, highly electropositive centres. This is due to the fact that oxygen is a more electronegative atom and thus prefers to bind via more ionic interactions. Examples of this type of coordination were presented in the work of Arnold et al. from 2015. The group found that actinide complexes of PCO involving
Coordination via the oxygen atom is favoured by hard, highly electropositive centres. This is due to the fact that oxygen is a more electronegative atom and thus prefers to bind via more ionic interactions. Examples of this type of coordination were presented in the work of Arnold et al. from 2015. The group found that actinide complexes of PCO involving uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly ...
and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
both coordinated through the oxygen. This is the result of the contracted nature of the actinide orbitals which makes the metal centres more 'core-like' thus favouring ionic interactions.
Attack by phosphorus
On the other hand, softer, more polarisable centres prefer to coordinate in a more covalent manner through the phosphorus atom. Examples of this include complexes accommodating a neutral or sparsely charged transition metal centre. The first example of this nature of PCO binding was published by Grutzmacher and co-workers in 2012. The group's studies used a Re(I) complex and the analysis of its bonding parameters and electronic structure showed that the phosphaethynolate anion coordinated in a bent fashion. This suggested the Re(I) – P bond possessed a highlycovalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
character thus the complex would be best described as a metallaphosphaketene. It wasn't until four years later that a second example of this coordination nature of PCO was identified. This time it came in the form of a W(0) pentacarbonyl complex produced by the Goicoechea group.

Rearrangement of coordination character
There is one particular reaction studied by Grutzmacher et al. that exhibits the rearrangement of coordination character of PCO. Initially when reacting the anion with triorganylsilicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
compounds, it binds via the oxygen forming the kinetic oxyphosphaalkyne product. The thermodynamic silyl phosphaketene product is generated when the kinetic product rearranges to allow PCO to coordinate through phosphorus.
The formation of the kinetic product is charged controlled and thus explains why it is formed by oxygen coordination. The oxygen atom favours a larger degree of ionic interactions as a result of its greater electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
. Contrastingly, the thermodynamic product of the reaction is generated under orbital control. This comes in the form of phosphorus coordination as the largest contribution in the HOMO
''Homo'' () is the genus that emerged in the (otherwise extinct) genus '' Australopithecus'' that encompasses the extant species ''Homo sapiens'' (modern humans), plus several extinct species classified as either ancestral to or closely relat ...
of the anion resides on the phosphorus atom; this is clearly visible in ''Figure 3''.
Reactivity of the anion
Extensive studies involving the phosphaethynolate anion have shown that it can react in a variety of ways. It has documented use in cycloadditions, as a phosphorus transfer agent, a synthetic building block and as pseudo halide ligands (''as described above'').Phosphorus transfer agents
In these types of reactions, CO is released as the phosphaethynolate anion acts as either a mildnucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
source of phosphorus or a Brønsted base. Examples of these types of reactions involving PCO include work conducted by Grutzmacher and Goicoechea.
In 2014, Grutzmacher et al. reported that an imidazolium salt would react with the phosphaethynolate anion to produce a phosphinidine carbene adduct. Computational mechanistic studies were conducted on this reaction using density functional theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-bo ...
at the B3LYP/6-31+G* level. The results of these investigations suggested that the lowest energy and therefore most likely pathway involves PCO acting as a Brønsted base initially deprotonating the acidic imidazolium cation to generate the intermediate phosphaketene, HPCO. The highly unstable protonated PCO remains hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
ed to the newly produced N-heterocylic carbene prior to rearrangement and formation of the observed product. In this case, PCO does not act as a mild nucleophile due to the augmented stability of the starting imidazolium cation.
On the other hand, in the work published by Goicoechea and co-workers in 2015, the phosphaethynolate anion can be seen to act as a source of nucleophilic phosphide ('). The anion was seen to add across the double bond of cyclotrisilene thus introducing a phosphorus vertex into its scaffold (after undergoing decarbonylation).

Cycloaddition Reagents
After synthesising the potassium salt of the phosphaethynolate anion in 2013 ''(see above)'', Goicoechea et al. began to look into the potential of PCO towardscycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". ...
s. They found that the anion could react in a +2fashion with a diphenyl ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound eth ...
to produce the first isolatable example of a four-membered monoanionic phosphorus containing heterocycle. They employed the same method to test other unsaturated substrates such as carbodiimides and found that the likelihood of cyclisation heavily relies on the nature of the substituents on the unsaturated substrate.
Cycloaddition reactions involving the phosphaethynolate anion have also been shown by Grutzmacher and co-workers to be a viable synthetic route to other heterocycles. One simple example is the reaction between the NaPCO and an α-pyrone. This reaction yields the sodium phosphinin-2-olate salt which is stable to both air and moisture.
Synthetic building blocks
A large part of the research involving PCO is now looking into utilising the anion as a synthetic building block to derive phosphorus containing analogues of small molecules. The first major breakthrough in this area came from Goicoechea et al. in 2013; they published the reaction between the PCO anion and ammonium salts which yielded the phosphorus containing analogue of urea in which phosphorus replaces a nitrogen atom. The group predict that this heavier congener could have applications in new materials, anion sensing and coordination chemistry. Goicoechea and co-workers were also able to isolate the heavily sought after phosphorus containing analogue of isocyanic acid, HPCO, in 2017. This molecule is thought to be a crucial intermediate in a lot of reactions involving PCO (including P-transfer to an imidazolium cation; ''see above''). Moreover, the most recent addition to this class of small molecules is the phosphorus containing analogue of N,N-dimethylformamide. This work in which the phosphorus again replaces a nitrogen atom was published in 2018 by Stephan and co-workers. Generating acylphosphines in this manner is considered a much milder route than other current strategies that require multi-step syntheses involvingtoxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
, volatile and pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
reagents.
Other analogues of PCO
The other analogues of the phosphaethynolate anion all obey the general formulae E-C-X and are made by varying E and X. When changing either atom, unique trends amongst the different analogues become apparent.
Varying E
As 'E' is varied by descending group 15, there is a clear shift in the weights of theresonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
structures towards the phosphaketene analogue . This reflects the decrease in effective orbital overlap between E and C which in turn disfavours multiple bond formation. This increasing tendency to form double and not triple E-C bonds is also reflected in calculated E-C bond lengths . The data from ''Table 1'' is evidence of E-C bond elongation which correlates with the change from triple to double bond.
In addition, NBO analysis highlights that the greatest electron delocalisation within the anions stems from the donation of an oxygen lone pair into the E−C π antibonding orbital. The energy value associated with this donation is seen to increase down the group . This explains the increasing resonance weight towards the ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound eth ...
like isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
...
as populating antibonding orbitals usually suggests the breaking of a bond.
The shift towards the ketene isomer will also cause an increase in charge density on the elemental 'E' atom; this makes the elemental atom an increasing source of nucleophilicity .

Varying X
 The simplest analogue that can be formed as 'X' is varied is . This anion was first isolated by Becker et al. by reacting the phosphaethynolate anion with carbon disulphide. Unlike PCO, PCS shows ambidentate nucleophilic tendencies towards the W(0) complex mentioned above.
This is the result of a reduced difference in electronegativity between E and X thus neither atom offers a substantial advantage over the other in terms of providing ionic contributions to bonding. As a result, the average electron density in PCS is spread over the entire anion whereas in PCO, most electron density is localised on the phosphorus atom as this is the atom which bonds to form the thermodynamically favourable product.
The simplest analogue that can be formed as 'X' is varied is . This anion was first isolated by Becker et al. by reacting the phosphaethynolate anion with carbon disulphide. Unlike PCO, PCS shows ambidentate nucleophilic tendencies towards the W(0) complex mentioned above.
This is the result of a reduced difference in electronegativity between E and X thus neither atom offers a substantial advantage over the other in terms of providing ionic contributions to bonding. As a result, the average electron density in PCS is spread over the entire anion whereas in PCO, most electron density is localised on the phosphorus atom as this is the atom which bonds to form the thermodynamically favourable product.
References
{{Reflist, 30em, refs= Quan, Z. J. and Wang, X. C. (2014) 'The 2-phosphaethynolate anion: Convenient synthesis and the reactivity', ''Organic Chemistry Frontiers''doi:10.1039/c4qo00189c
Camp, C., Settineri, N., Lefèvre, J., Jupp, A. R., Goicoechea, J. M., Maron, L. and Arnold, J. (2015) 'Uranium and thorium complexes of the phosphaethynolate ion', ''Chemical Science''
doi: 10.1039/c5sc02150b
Heift, D., Benko, Z. and Grützmacher, H. (2014) 'Is the phosphaethynolate anion, (OCP)-, an ambident nucleophile? A spectroscopic and computational study', ''Dalton Transactions''
doi: 10.1039/c3dt53569j
Jupp, A. R., and Goicoechea, J. M. (2013) 'Phosphinecarboxamide: A Phosphorus-Containing Analogue of Urea and Stable Primary Phosphine', ''J. Am. Chem. Soc.''
DOI:10.1021/ja4115693
Becker, G., Schwarz, W., Seidler, N. and Westerhausen, M. (1992) 'Acyl‐ und Alkylidenphosphane. XXXIII. Lithoxy‐methylidenphosphan · DME und ‐methylidinphosphan · 2 DME — Synthese und Struktur', ''ZAAC ‐ Journal of Inorganic and General Chemistry''
doi: 10.1002/zaac.19926120113
Grutzmacher, H., and Goicoechea, J. (2018) 'The chemistry of the 2‐phosphaethynolate anion', ''Angew. Chem. Int. Ed''. doi
10.1002/anie.201803888
Pyykkö, P. (2015) 'Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: A summary', ''Journal of Physical Chemistry A''
doi: 10.1021/jp5065819
Westerhausen, M., Schneiderbauer, S., Piotrowski, H., Suter, M. and Nöth, H. (2002) 'Synthesis of alkaline earth metal bis(2-phosphaethynolates)', ''Journal of Organometalic Chemistry''
doi: 10.1016/S0022-328X(01)01267-0
Puschmann, F. F., Stein, D., Heift, D., Hendriksen, C., Gal, Z. A., Grützmacher, H. F. and Grützmacher, H. (2011) 'Phosphination of carbon monoxide: A simple synthesis of sodium phosphaethynolate (NaOCP)', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201102930
Jupp, A. R. and Goicoechea, J. M. (2013) 'The 2-phosphaethynolate anion: A convenient synthesis and +2cycloaddition chemistry', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201305235
Hou, G. L., Chen, B., Transue, W. J., Yang, Z., Grützmacher, H., Driess, M., Cummins, C. C., Borden, W. T. and Wang, X. Bin (2017) 'Spectroscopic Characterization, Computational Investigation, and Comparisons of ECX-(E = As, P, and N; X = S and O) Anions', ''Journal of the American Chemical Society''
doi: 10.1021/jacs.7b02984
Alidori, S., Heift, D., Santiso-Quinones, G., Benkå, Z., Grützmacher, H., Caporali, M., Gonsalvi, L., Rossin, A. and Peruzzini, M. (2012) 'Synthesis and characterization of terminal e(XCO)(CO) 2(triphos)(X=N, P): Isocyanate versus phosphaethynolate complexes', ''Chemistry - A European Journal''
doi: 10.1002/chem.201202590
Jupp, A. R., Geeson, M. B., McGrady, J. E. and Goicoechea, J. M. (2016) 'Ambient-Temperature Synthesis of 2-Phosphathioethynolate, PCS-, and the Ligand Properties of ECX-(E = N, P; X = O, S)', ''European Journal of Inorganic Chemistry''
doi: 10.1002/ejic.201501075
Robinson, T. P., Cowley, M. J., Scheschkewitz, D. and Goicoechea, J. M. (2015) 'Phosphide delivery to a cyclotrisilene', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201409908
Tambornino, F., Hinz, A., Köppe, R. and Goicoechea, J. M. (2018) 'A General Synthesis of Phosphorus- and Arsenic-Containing Analogues of the Thio- and Seleno-cyanate Anions', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201805348
Lu, Y., Wang, H., Xie, Y., Liu, H. and Schaefer, H. F. (2014) 'The cyanate and 2-phosphaethynolate anion congeners ECO-(E = N, P, As, Sb, Bi): Prelude to experimental characterization', ''Inorganic Chemistry''
doi: 10.1021/ic500780h
Szkop, K., Jupp, A. R., and Stephan, D. W. (2018) 'P,P‑Dimethylformylphosphine: The Phosphorus Analogue of N,N‑Dimethylformamide' ''J. Am. Chem. Soc''
doi:10.1021/jacs.8b09266
Chen, X., Alidori, S., Puschmann, F. F., Santiso-Quinones, G., Benko, Z., Li, Z., Becker, G., Grützmacher, H. F. and Grützmacher, H. (2014) 'Sodium phosphaethynolate as a building block for heterocycles', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201308220
Hinz, A., Labbow, R., Rennick, C., Schulz, A. and Goicoechea, J. M. (2017) 'HPCO—A Phosphorus-Containing Analogue of Isocyanic Acid', ''Angewandte Chemie - International Edition''
doi: 10.1002/anie.201700368
Tondreau, A. M., Benko, Z., Harmer, J. R. and Grützmacher, H. (2014) 'Sodium phosphaethynolate, Na(OCP), as a “p” transfer reagent for the synthesis of N-heterocyclic carbene supported P3 and PAsP radicals', ''Chemical Science''
doi: 10.1039/c3sc53140f
Anions Organophosphorus compounds Physical organic chemistry Substances discovered in the 1990s