Perovskite (structure) on:
[Wikipedia]
[Google]
[Amazon]

 A perovskite is a crystalline material of formula ABX3 with a
A perovskite is a crystalline material of formula ABX3 with a
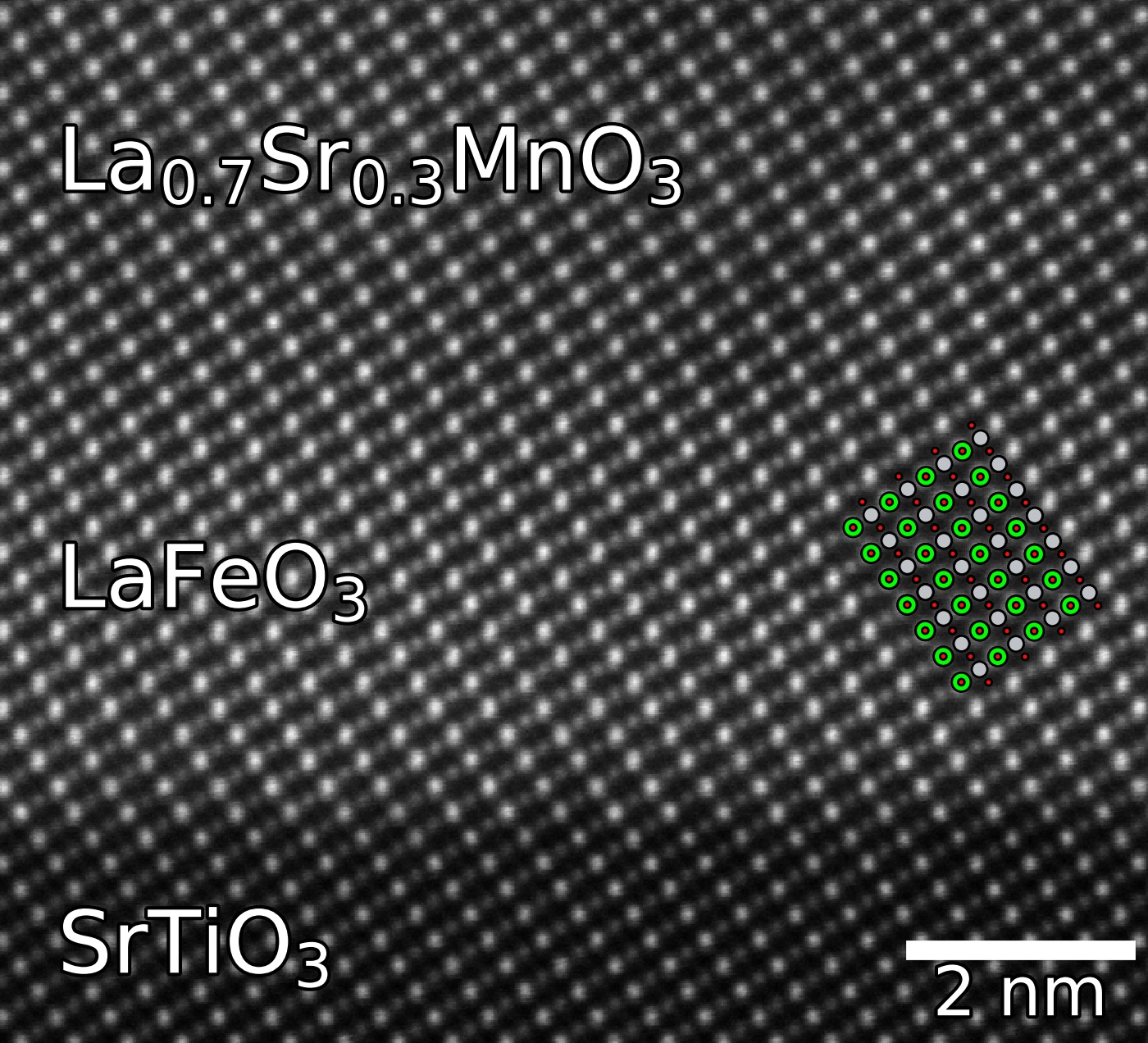 Perovskites can be deposited as epitaxial thin films on top of other perovskites, using techniques such as
Perovskites can be deposited as epitaxial thin films on top of other perovskites, using techniques such as
 The notation consists of a letter a/b/c, which describes the rotation around a Cartesian axis and a superscript +/—/0 to denote the rotation with respect to the adjacent layer. A "+" denotes that the rotation of two adjacent layers points in the same direction, whereas a "—" denotes that adjacent layers are rotated in opposite directions. Common examples are a0a0a0, a0a0a– and a0a0a+ which are visualized here.
The notation consists of a letter a/b/c, which describes the rotation around a Cartesian axis and a superscript +/—/0 to denote the rotation with respect to the adjacent layer. A "+" denotes that the rotation of two adjacent layers points in the same direction, whereas a "—" denotes that adjacent layers are rotated in opposite directions. Common examples are a0a0a0, a0a0a– and a0a0a+ which are visualized here.
on

 Of interest in the context of solar energy are materials of the type . Thus, the quat cation occupies the B site and the metals occupy the A sites.These materials are the basis of perovskite solar cells. These materials have high
Of interest in the context of solar energy are materials of the type . Thus, the quat cation occupies the B site and the metals occupy the A sites.These materials are the basis of perovskite solar cells. These materials have high
Superionic Conductivity in Lithium-Rich Anti-Perovskites
Lattice and Magnetic and Electronic Transport Properties in Antiperovskite Compounds
with which the structure can be interactively rotated)
{{DEFAULTSORT:Perovskite (Structure) Mineralogy Solar power * Crystal structure types Crystallography Materials science de:Perowskit#Kristallstruktur

 A perovskite is a crystalline material of formula ABX3 with a
A perovskite is a crystalline material of formula ABX3 with a crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
similar to that of the mineral perovskite, this latter consisting of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia
Russia, or the Russian Federation, is a country spanning Eastern Europe and North Asia. It is the list of countries and dependencies by area, largest country in the world, and extends across Time in Russia, eleven time zones, sharing Borders ...
by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). In addition to being one of the most abundant structural families, perovskites have wide-ranging properties and applications.
Structure
Perovskite structures are adopted by many compounds that have the chemical formula ABX3. 'A' and 'B' are positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by anoctahedron
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of i ...
of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where both/either the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states. The idealized form is a cubic structure (space group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that ...
Pmm, no. 221), which is rarely encountered. The orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, res ...
(e.g. space group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that ...
Pnma, no. 62, or Amm2, no. 38) and tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
(e.g. space group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that ...
I4/mcm, no. 140, or P4mm, no. 99) structures are the most common non-cubic variants. Although the perovskite structure is named after CaTiO3, this mineral has a non-cubic structure. SrTiO3 and CaRbF3 are examples of cubic perovskites. Barium titanate
Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. It is the barium salt of metatitanic acid. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a Ferroelectricity, ferroe ...
is an example of a perovskite which can take on the rhombohedral (space group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that ...
R3m, no. 160), orthorhombic, tetragonal and cubic forms depending on temperature.
In the idealized cubic unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector
In mathematics, a unit vector i ...
of such a compound, the type 'A' atom sits at cube corner position (0, 0, 0), the type 'B' atom sits at the body-center position (1/2, 1/2, 1/2) and X atoms (typically oxygen) sit at face centered positions (1/2, 1/2, 0), (1/2, 0, 1/2) and (0, 1/2, 1/2). The diagram to the right shows edges for an equivalent unit cell with A in the cube corner position, B at the body center, and X at face-centered positions.
Four general categories of cation-pairing are possible: A+B2+X−3, or 1:2 perovskites; A2+B4+X2−3, or 2:4 perovskites; A3+B3+X2−3, or 3:3 perovskites; and A+B5+X2−3, or 1:5 perovskites.
The relative ion size requirements for stability of the cubic structure are quite stringent, so slight buckling and distortion can produce several lower-symmetry distorted versions, in which the coordination numbers of A cations, B cations or both are reduced. Tilting of the BO6 octahedra reduces the coordination of an undersized A cation from 12 to as low as 8. Conversely, off-centering of an undersized B cation within its octahedron allows it to attain a stable bonding pattern. The resulting electric dipole is responsible for the property of ferroelectricity and shown by perovskites such as BaTiO3 that distort in this fashion.
Complex perovskite structures contain two different B-site cations. This results in the possibility of ordered and disordered variants.
Defect perovskites
Rhenium trioxide is a simple example of a defect perovskite: the central atom found in classical perovskites is absent., 144px Also common are the defect perovskites. Instead of the ideal ABO3 stoichiometry, defect perovskites are missing some or all of the A, B, or O atoms. One example is rhenium trioxide. It is missing the A atoms. Uranium trihydride is another example of a simple defect perovskite. Here, all B sites are vacant, H− occupies the O sites, and the large U3+ ion occupies the A site. Many high temperature superconductors, especiallycuprate superconductor
Cuprate superconductors are a family of High-temperature superconductivity, high-temperature superconducting materials made of layers of copper oxides () alternating with layers of other metal oxides, which act as charge reservoirs. At ambient p ...
, adopt defect perovskite structures. The prime example is yttrium barium copper oxide
Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen ...
(YBCO), which has the formula YBa2Cu3O7. In this material Y3+ and Ba2+, which are relatively large, occupy all A sites. Cu occupies all B sites. Two O atoms per formula unit are absent, hence the term ''defect''. The compound YBa2Cu3O7 is a superconductor. The average oxidation state of copper is Cu(7/3)+ since Y3+ and Ba2+ have fixed oxidation states. When heated in the absence of O2, the solid loses its superconducting properties, relaxes to the stoichiometry YBa2Cu3O6.5, and all copper sites convert to Cu2+. The material thus is an oxygen carrier, shuttling between two defect perovskites:
:
Layered perovskites
 Perovskites can be deposited as epitaxial thin films on top of other perovskites, using techniques such as
Perovskites can be deposited as epitaxial thin films on top of other perovskites, using techniques such as pulsed laser deposition
Pulsed laser deposition (PLD) is a physical vapor deposition (PVD) technique where a high-power pulsed laser beam is focused inside a vacuum chamber to strike a target of the material that is to be deposited. This material is vaporized from the ...
and molecular-beam epitaxy
Molecular-beam epitaxy (MBE) is an epitaxy method for thin-film deposition of single crystals. MBE is widely used in the manufacture of semiconductor devices, including transistors. MBE is used to make diodes and MOSFETs (MOS field-effect transis ...
. These films can be a couple of nanometres thick or as small as a single unit cell.
Perovskites may be structured in layers, with the structure separated by thin sheets of intrusive material. Based on the chemical makeup of their intrusions, these layered phases can be defined as follows:
* Aurivillius phase: the intruding layer is composed of a []2+ ion, occurring every ''n'' layers, leading to an overall chemical formula of []-. Their oxide ion-conducting properties were first discovered in the 1970s by Takahashi et al., and they have been used for this purpose ever since.
* Dion−Jacobson phase: the intruding layer is composed of an alkali metal (M) every ''n'' layers, giving the overall formula as
* Ruddlesden-Popper phase: the simplest of the phases, the intruding layer occurs between every one (''n'' = 1) or multiple (''n'' > 1) layers of the lattice. Ruddlesden−Popper phases have a similar relationship to perovskites in terms of atomic radii of elements with A typically being large (such as La or Sr) with the B ion being much smaller typically a transition metal (such as Mn, Co or Ni).
Complex perovskites
Although there is a large number of simple known ABX3 perovskites, this number can be greatly expanded if the A and B sites are increasingly doubled / complex ABX6. Ordered double perovskites are usually denoted as A2BO6 where disordered are denoted as A(B)O3. In ordered perovskites, three different types of ordering are possible: rock-salt, layered, and columnar. The most common ordering is rock-salt followed by the much more uncommon disordered and very distant columnar and layered. The formation of rock-salt superstructures is dependent on the B-site cation ordering. Octahedral tilting can occur in double perovskites, however Jahn–Teller distortions and alternative modes alter the B–O bond length.Antiperovskites
The lattice of an antiperovskites (or inverse perovskites) is the same as that of the perovskite structure, but the anion and cation positions are switched. The typical perovskite structure is represented by the general formula ABX3, where A and B are cations and X is an anion. When the anion is the (divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
) oxide ion, A and B cations can have charges 1 and 5, respectively, 2 and 4, respectively, or 3 and 3, respectively. In antiperovskite compounds, the general formula is reversed, so that the X sites are occupied by an electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
ion, i.e., cation (such as an alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
), while A and B sites are occupied by different types of anion. In the ideal cubic cell, the A anion is at the corners of the cube, the B anion at the octahedral
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of i ...
center, and the X cation is at the faces of the cube. Thus the A anion has a coordination number of 12, while the B anion sits at the center of an octahedron with a coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
of 6. Similar to the perovskite structure, most antiperovskite compounds are known to deviate from the ideal cubic structure, forming orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, res ...
or tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
phases depending on temperature and pressure.
Whether a compound will form an antiperovskite structure depends not only on its chemical formula, but also the relative sizes of the ionic radii of the constituent atoms. This constraint is expressed in terms of the Goldschmidt tolerance factor, which is determined by the radii, ra, rb and rx, of the A, B, and X ions.
Tolerance factor =
For the antiperovskite structure to be structurally stable, the tolerance factor must be between 0.71 and 1. If between 0.71 and 0.9, the crystal will be orthorhombic or tetragonal. If between 0.9 and 1, it will be cubic. By mixing the B anions with another element of the same valence but different size, the tolerance factor can be altered. Different combinations of elements result in different compounds with different regions of thermodynamic stability for a given crystal symmetry..
Examples
Antiperovskites naturally occur in sulphohalite, galeite, schairerite, kogarkoite, nacaphite, arctite, polyphite, and hatrurite. It is also demonstrated in superconductive compounds such as CuNNi3 and ZnNNi3. Discovered in 1930, metallic antiperovskites have the formula M3AB where M represents a magnetic element, Mn, Ni, or Fe; A represents a transition or main group element, Ga, Cu, Sn, and Zn; and B represents N, C, or B. These materials exhibitsuperconductivity
Superconductivity is a set of physical properties observed in superconductors: materials where Electrical resistance and conductance, electrical resistance vanishes and Magnetic field, magnetic fields are expelled from the material. Unlike an ord ...
, giant magnetoresistance, and other unusual properties.
Antiperovskite manganese nitrides exhibit zero thermal expansion
Thermal expansion is the tendency of matter to increase in length, area, or volume, changing its size and density, in response to an increase in temperature (usually excluding phase transitions).
Substances usually contract with decreasing temp ...
.
Octahedral tilting
Beyond the most common perovskite symmetries (cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
, tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
, orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, res ...
), a more precise determination leads to a total of 23 different structure types that can be found. These 23 structure can be categorized into 4 different so-called tilt systems that are denoted by their respective Glazer notation.
 The notation consists of a letter a/b/c, which describes the rotation around a Cartesian axis and a superscript +/—/0 to denote the rotation with respect to the adjacent layer. A "+" denotes that the rotation of two adjacent layers points in the same direction, whereas a "—" denotes that adjacent layers are rotated in opposite directions. Common examples are a0a0a0, a0a0a– and a0a0a+ which are visualized here.
The notation consists of a letter a/b/c, which describes the rotation around a Cartesian axis and a superscript +/—/0 to denote the rotation with respect to the adjacent layer. A "+" denotes that the rotation of two adjacent layers points in the same direction, whereas a "—" denotes that adjacent layers are rotated in opposite directions. Common examples are a0a0a0, a0a0a– and a0a0a+ which are visualized here.
Examples
Minerals
Aside fromperovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as , known as the perovskite (stru ...
itself, some perovskite minerals include loparite and bridgmanite.Bridgemaniteon
Mindat.org
Mindat.org is a non-commercial interactive online database covering minerals around the world. Originally created by Jolyon Ralph as a private project in 1993, it was launched as a community-editable website in October 2000. it is operated by ...
Bridgmanite is a silicate with the chemical formula . It is the most common mineral in the Earth's mantle. At high pressures associated with the deeper mantel, the Si sites feature octahedral units.
At the high pressure conditions of the Earth's lower mantle, the pyroxene
The pyroxenes (commonly abbreviated Px) are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents ions of calcium (Ca), sodium (Na), iron ( ...
enstatite, MgSiO3, which otherwise has tetrahedral Si sites, transforms into a denser perovskite-structured polymorph; this phase may be the most common mineral in the Earth. This phase has the orthorhombically distorted perovskite structure (GdFeO3-type structure) that is stable at pressures from ~24 GPa to ~110 GPa. However, it cannot be transported from depths of several hundred km to the Earth's surface without transforming back into less dense materials. At higher pressures, MgSiO3 perovskite, commonly known as silicate perovskite, transforms to post-perovskite.
Inorganic perovskites lacking oxygen
Although the most common perovskite compounds contain oxygen, there are a few perovskite compounds that form without oxygen. Fluoride perovskites such as NaMgF3 are well known. A large family of metallic perovskite compounds can be represented by RT3M (R: rare-earth or other relatively large ion, T: transition metal ion and M: light metalloids). The metalloids occupy the octahedrally coordinated "B" sites in these compounds. RPd3B, RRh3B and CeRu3C are examples. MgCNi3 is a metallic perovskite compound and has received lot of attention because of its superconducting properties. An even more exotic type of perovskite is represented by the mixed oxide-aurides of Cs and Rb, such as Cs3AuO, which contain large alkali cations in the traditional "anion" sites, bonded to O2− and Au− anions.Organic perovskites

 Of interest in the context of solar energy are materials of the type . Thus, the quat cation occupies the B site and the metals occupy the A sites.These materials are the basis of perovskite solar cells. These materials have high
Of interest in the context of solar energy are materials of the type . Thus, the quat cation occupies the B site and the metals occupy the A sites.These materials are the basis of perovskite solar cells. These materials have high charge carrier
In solid state physics, a charge carrier is a particle or quasiparticle that is free to move, carrying an electric charge, especially the particles that carry electric charges in electrical conductors. Examples are electrons, ions and holes. ...
mobility and charge carrier lifetime that allow light-generated electrons and holes to move far enough to be extracted as current, instead of losing their energy as heat within the cell.
Applications, real and aspirational
Probably the dominant applications of perovskites are in microelectronics andtelecommunications
Telecommunication, often used in its plural form or abbreviated as telecom, is the transmission of information over a distance using electronic means, typically through cables, radio waves, or other communication technologies. These means of ...
, which exploit the ferroelectric properties of barium titanate
Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. It is the barium salt of metatitanic acid. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a Ferroelectricity, ferroe ...
, lithium niobate
Lithium niobate () is a synthetic salt consisting of niobium, lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperatur ...
, lead zirconium titanate and others.
Physical properties of interest to materials science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.
The intellectual origins of materials sci ...
among perovskites They are applicable to lasers. They are also some interests for scintillator as they have a large light yield for radiation conversion. Because of the flexibility of bond angles inherent in the perovskite structure there are many different types of distortions that can occur from the ideal structure. These include tilting of the octahedra, displacements of the cations out of the centers of their coordination polyhedra, and distortions of the octahedra driven by electronic factors ( Jahn-Teller distortions). The financially biggest application of perovskites is in ceramic capacitors, in which BaTiO3 is used because of its high dielectric constant. Light-emitting diodes exploit the high photoluminescence quantum efficiencies of perovskites. In the area of photoelectrolysis, water electrolysis at 12.3% efficiency can use perovskite photovoltaics. Scintillators based on cerium-doped lutetium aluminum perovskite (LuAP:Ce) single crystals were reported. Layered Ruddlesden-Popper perovskites have shown potential as fast novel scintillators with room temperature light yields up to 40,000 photons/MeV, fast decay times below 5 ns and negligible afterglow. In addition this class of materials have shown capability for wide-range particle detection, including alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s and thermal neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s.
See also
* Antiperovskite * Aurivillius phases * Diamond anvil * Goldschmidt tolerance factor * Ruddlesden-Popper phase *Spinel
Spinel () is the magnesium/aluminium member of the larger spinel group of minerals. It has the formula in the cubic crystal system. Its name comes from the Latin word , a diminutive form of ''spine,'' in reference to its pointed crystals.
Prop ...
References
Further reading
* *Superionic Conductivity in Lithium-Rich Anti-Perovskites
Lattice and Magnetic and Electronic Transport Properties in Antiperovskite Compounds
External links
* (includeswith which the structure can be interactively rotated)
{{DEFAULTSORT:Perovskite (Structure) Mineralogy Solar power * Crystal structure types Crystallography Materials science de:Perowskit#Kristallstruktur