Passerini Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Passerini reaction is a 
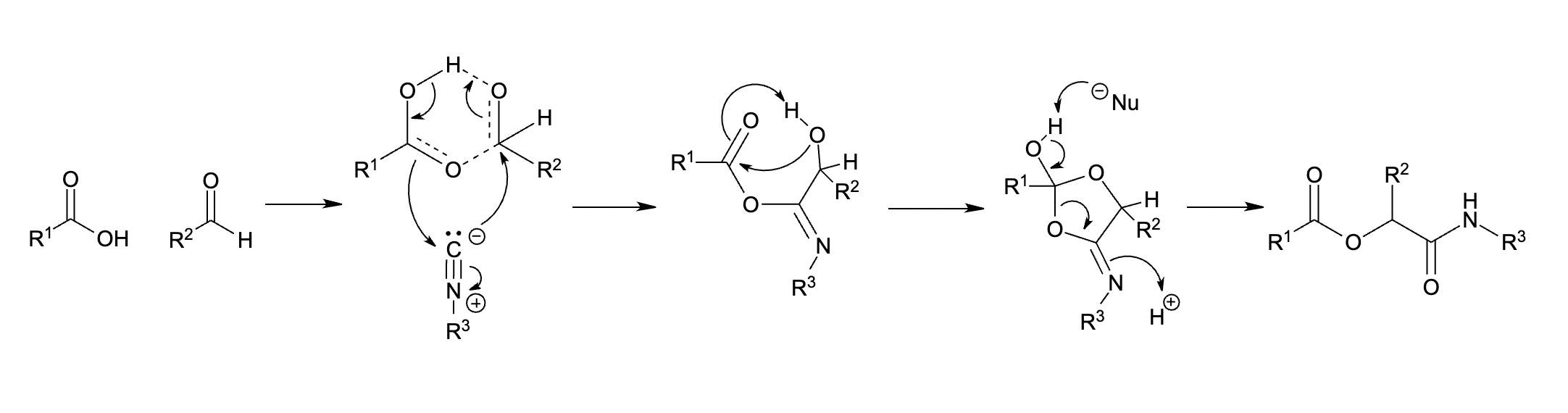 This mechanism involves a trimolecular reaction between the isocyanide, carboxylic acid, and carbonyl in a sequence of
This mechanism involves a trimolecular reaction between the isocyanide, carboxylic acid, and carbonyl in a sequence of
 In polar solvents, such as
In polar solvents, such as
 The original Passerini reaction produces acyclic
The original Passerini reaction produces acyclic
 This reaction has also been used for polymerization,
This reaction has also been used for polymerization,

chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
involving an isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragme ...
, an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
(or ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
), and a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
to form a α- acyloxy amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
. This addition reaction is one of the oldest isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragme ...
-based multicomponent reactions and was first described in 1921 by Mario Passerini in Florence, Italy. It is typically carried out in aprotic
A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hydr ...
solvents but can alternatively be performed in water, ionic liquids, or deep eutectic solvent
Deep eutectic solvents or DESs are solutions of Lewis or Brønsted acids and bases which form a eutectic mixture. Deep eutectic solvents are highly tunable through varying the structure or relative ratio of parent components and thus have a wide ...
s. It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial
Combinatorics is an area of mathematics primarily concerned with counting, both as a means and as an end to obtaining results, and certain properties of finite structures. It is closely related to many other areas of mathematics and has many ...
and medicinal chemistry
Medicinal or pharmaceutical chemistry is a scientific discipline at the intersection of chemistry and pharmacy involved with drug design, designing and developing pharmaceutical medication, drugs. Medicinal chemistry involves the identification, ...
with recent utility in green chemistry
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. Wh ...
and polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applic ...
. As isocyanides exhibit high functional group tolerance, chemoselectivity Chemoselectivity is the preferential reaction of a chemical reagent with one of two or more different functional groups.
In a chemoselective system, a reagent in the presence of an aldehyde and an ester would mostly target the aldehyde, even if it ...
, regioselectivity
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
, and stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
, the Passerini reaction has a wide range of synthetic applications.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 Mechanism
The Passerini reaction has been hypothesized to occur through two mechanistic pathways. The reaction pathways are dependent on the solvent used.Concerted mechanism
A concerted mechanism, seen in SN2 and Diels−Alder reactions, is theorized to occur when the Passerini reagents are present at high concentration inaprotic
A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hydr ...
solvents.
 This mechanism involves a trimolecular reaction between the isocyanide, carboxylic acid, and carbonyl in a sequence of
This mechanism involves a trimolecular reaction between the isocyanide, carboxylic acid, and carbonyl in a sequence of nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
s. The reaction proceeds first through an imidate
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between an imidic acid () and an Alcohol (chemistry), alcohol, with the general formula .
They are also known as imino ethers, since they ...
intermediate and then undergoes Mumm rearrangement The Mumm rearrangement is an organic reaction and a rearrangement reaction. It describes a 1,3(O-N) acyl transfer of an acyl imidate or isoimide group to an imide.
The reaction is of relevance as part of the Ugi reaction
In organic chemistry, ...
to afford the Passerini product.
As the Mumm rearrangement The Mumm rearrangement is an organic reaction and a rearrangement reaction. It describes a 1,3(O-N) acyl transfer of an acyl imidate or isoimide group to an imide.
The reaction is of relevance as part of the Ugi reaction
In organic chemistry, ...
requires a second carboxylic acid molecule, this mechanism classifies the Passerini reaction as an organocatalytic reaction.
Ionic mechanism
 In polar solvents, such as
In polar solvents, such as methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
or water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
, the carbonyl is protonated before nucleophilic addition of the isocyanide, affording a nitrilium ion intermediate. This is followed by the addition of a carboxylate, acyl group transfer and proton transfer respectively to give the desired Passerini product.
Reaction control
Molecular weights of polymers synthesized through the Passerini can be controlled through stoichiometric means. For example, polymer chain length and weight can adjusted through isocyanide stoichiometry, and polymer geometry can be influenced through starting reagents. To facilitate the Passerini reaction between bulky, sterically hindered reagents, a vortex fluidic device can be used to induce high shear conditions. These conditions emulate the effects of high temperature and pressure, allowing the Passerini reaction to proceed fairly quickly. The Passerini reaction can also exhibit enantioselectivity. Addition of tert-butyl isocyanide to a wide variety of aldehydes (aromatic, heteroaromatic, olefinic, acetylenic, aliphatic) is achieved using a catalytic system of tetrachloride and a chiral bisphosphoramide which provides good yield and good enantioselectivities. For other types of isocyanides, rate of addition of isocyanide to reaction mixture dictates good yields and high selectivities.Applications
Apart from forming α- acyloxyamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
products, the Passerini reaction can be used to form heterocycles, polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s, amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s, and medicinal
Medicine is the science and Praxis (process), practice of caring for patients, managing the Medical diagnosis, diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, ...
products.
Heterocycles
depsipeptide A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR-. Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure result ...
s which are labile in physiological conditions. To increase product stability for medicinal use, post-Passerini cyclization reactions have been used to afford heterocycles such as β-lactams, butenolide
Butenolides are a class of lactones with a four-carbon heterocycle, heterocyclic ring structure.Joule JA, Mills K. (2000). Heterocyclic Chemistry 4th ed. Blackwell Science Publishing: Oxford, UK They are sometimes considered redox, oxidized deri ...
s, and isocoumarin
Isocoumarin (1''H''-2-benzopyran-1-one; 3,4-benzo-2-pyrone) is a lactone, a type of natural organic compound.
Known natural compounds
* Thunberginol A and B
; dihydroisocoumarins
* Hydrangenol
* Phyllodulcin
* Thunberginol C, D, E and G ...
s. To enable these cyclizations, reagents are pre-functionalized with reactive groups (ex. halogens, azides, etc.) and used in tandem with other reactions (ex. Passerini- Knoevenagel, Passerini- Dieckmann) to afford heterocyclic products. Compounds like three membered oxirane and aziridine derivatives, four-membered b-lactam
A lactam is a Cyclic compound, cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words ''lactone'' + ''amide''.
Nomenclature
Greek_alphabet#Letters, Greek prefixes in alpha ...
s, and five-membered tetrasubstituted 4,5-dihydropyrazole
Pyrazole is an organic compound with the chemical formula, formula . It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in Arene substitution pattern, ortho-substi ...
s have been produced through this reaction.
Polymers
 This reaction has also been used for polymerization,
This reaction has also been used for polymerization, monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
formation, and post-polymerization modification. The Passerini reaction has also been used to form sequence-defined polymers. Bifunctional
In chemistry, bifunctionality or difunctionality is the presence of two functional groups in a molecule. A bifunctional species has the properties of each of the two types of functional groups, such as an alcohol (), amide (), aldehyde (), nitrile ...
substrates can be used to undergo post-polymerization modification or serve as precursors for polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
. As this reaction has high functional group tolerance, the polymers created using this reaction are widely diverse with tuneable properties
Property is the ownership of land, resources, improvements or other tangible objects, or intellectual property.
Property may also refer to:
Philosophy and science
* Property (philosophy), in philosophy and logic, an abstraction characterizing an ...
. Macromolecules that have been produced with this reaction include macroamides, macrocyclic depsipeptides, three-component dendrimer
Dendrimers are highly ordered, Branching (polymer chemistry), branched molecules, polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a sph ...
s and three-armed star branched mesogen
A mesogen is a compound that displays liquid crystal properties. Mesogens can be described as disordered solids or ordered liquids because they arise from a unique state of matter that exhibits both solid- and liquid-like properties called the liq ...
core molecules.
Amino acids and pharmaceuticals
Passerini reaction has been employed for the formation of structures like α-amino acids, α-hydroxy-β-amino acids, α-ketoamides, β-ketoamides, α-hydroxyketones
In organic chemistry, a hydroxy ketone (often referred to simply as a ketol) is a functional group consisting of a ketone () flanked by a hydroxyl group (). Chemicals in this group can be classified by the position of the hydroxyl relative to the ...
and α-aminoxyamides. The Passerini reaction has synthesized α-Acyloxy carboxamides that have demonstrated activity as anti-cancer medications along with functionalized 60fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
s used in medicinal and plant chemistry. This reaction has also been used as a synthetic step in the total synthesis of commercially available pharmaceuticals such as telaprevir
Telaprevir (VX-950), marketed under the brand names Incivek and Incivo, is a pharmaceutical drug for the treatment of hepatitis C co-developed by Vertex Pharmaceuticals and Johnson & Johnson. It is a member of a class of antiviral drugs known as ...
(VX-950), an antiviral
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are a class of antimicrobials ...
sold by Vertex Pharmaceuticals and Johnson & Johnson.
See also
*Ugi reaction
In organic chemistry, the Ugi reaction is a multi-component reaction involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide.
The reaction is named after Ivar Karl Ugi, who first reported this reaction in ...
References
{{Reflist Carbon-carbon bond forming reactions Multiple component reactions Name reactions Amide synthesis reactions