PDZ Domain on:
[Wikipedia]
[Google]
[Amazon]
The PDZ domain is a common
 PDZ domain structure is partially conserved across the various proteins that contain them. They usually have 5-6 β-strands and one short and one long α-helix. Apart from this conserved fold, the
PDZ domain structure is partially conserved across the various proteins that contain them. They usually have 5-6 β-strands and one short and one long α-helix. Apart from this conserved fold, the
 The first discovered function of the PDZ domains was to anchor receptor proteins in the membrane to cytoskeletal components. PDZ domains also have regulatory functions on different signaling pathways. Any protein may have one or several PDZ domains, which can be identical or unique (see figure to right). This variety allows these proteins to be very versatile in their interactions. Different PDZ domains in the same protein can have different roles, each binding a different part of the target protein or a different protein altogether.
The first discovered function of the PDZ domains was to anchor receptor proteins in the membrane to cytoskeletal components. PDZ domains also have regulatory functions on different signaling pathways. Any protein may have one or several PDZ domains, which can be identical or unique (see figure to right). This variety allows these proteins to be very versatile in their interactions. Different PDZ domains in the same protein can have different roles, each binding a different part of the target protein or a different protein altogether.
 Another post-translational modification that can regulate PDZ domains is the formation of disulfide bridges. Many PDZ domains contain
Another post-translational modification that can regulate PDZ domains is the formation of disulfide bridges. Many PDZ domains contain
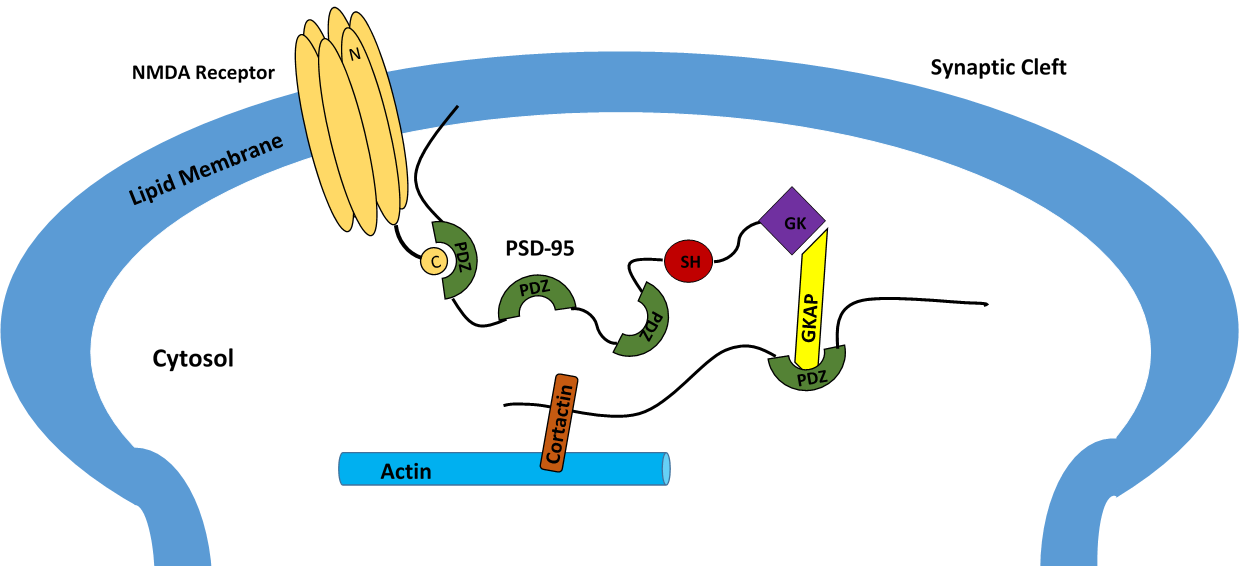 PSD-95 is a brain synaptic protein with three PDZ domains, each with unique properties and structures that allow PSD-95 to function in many ways. In general, the first two PDZ domains interact with receptors and the third interacts with cytoskeleton-related proteins. The main receptors associated with PSD-95 are NMDA receptors. The first two PDZ domains of PSD-95 bind to the C-terminus of NMDA receptors and anchor them in the membrane at the point of neurotransmitter release. The first two PDZ domains can also interact in a similar fashion with Shaker-type K+ channels. A PDZ interaction between PSD-95,
PSD-95 is a brain synaptic protein with three PDZ domains, each with unique properties and structures that allow PSD-95 to function in many ways. In general, the first two PDZ domains interact with receptors and the third interacts with cytoskeleton-related proteins. The main receptors associated with PSD-95 are NMDA receptors. The first two PDZ domains of PSD-95 bind to the C-terminus of NMDA receptors and anchor them in the membrane at the point of neurotransmitter release. The first two PDZ domains can also interact in a similar fashion with Shaker-type K+ channels. A PDZ interaction between PSD-95,
The PDZ Domain as a Complex Adaptive System
A concise technical summary and a statement of principal findings and ramifications of the PDZ Domain as a Complex Adaptive System
NCBI conserved domains entry
https://www.pdznet.eu
- An EU project to advance our understanding of the cellular signaling pathways and therapeutic potential of proteins comprising PDZ domains in healthy and pathological conditions such as cancer and neurological diseases. {{Protein domains Protein domains Membrane proteins
structural domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of se ...
of 80-90 amino-acids found in the signaling proteins of bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
, yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to consti ...
, plants, virus
A virus is a wikt:submicroscopic, submicroscopic infectious agent that replicates only inside the living Cell (biology), cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and ...
es and animal
Animals are multicellular, eukaryotic organisms in the Kingdom (biology), biological kingdom Animalia. With few exceptions, animals Heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, are Motilit ...
s. Proteins containing PDZ domains play a key role in anchoring receptor proteins in the membrane to cytoskeletal components. Proteins with these domains help hold together and organize signaling complexes at cellular membranes. These domains play a key role in the formation and function of signal transduction complexes. PDZ domains also play a highly significant role in the anchoring of cell surface receptors
Cell surface receptors (membrane receptors, transmembrane receptors) are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving (binding to) extracellular molecules. They are specialized integr ...
(such as Cftr and FZD7) to the actin
Actin is a protein family, family of Globular protein, globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in myofibril, muscle fibrils. It is found in essentially all Eukaryote, eukaryotic cel ...
cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is comp ...
via mediators like NHERF and ezrin.
''PDZ'' is an initialism
An acronym is a word or name formed from the initial components of a longer name or phrase. Acronyms are usually formed from the initial letters of words, as in ''NATO'' (''North Atlantic Treaty Organization''), but sometimes use syllables, as ...
combining the first letters of the first three proteins discovered to share the domain — post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein (zo-1). PDZ domains have previously been referred to as DHR (Dlg homologous region) or GLGF (glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
- leucine-glycine- phenylalanine) domains.
In general PDZ domains bind to a short region of the C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein i ...
of other specific proteins. These short regions bind to the PDZ domain by beta sheet augmentation. This means that the beta sheet in the PDZ domain is extended by the addition of a further beta strand from the tail of the binding partner protein. The C-terminal carboxylate group is bound by a nest (protein structural motif) in the PDZ domain.
Origins of discovery
PDZ is an acronym derived from the names of the first proteins in which the domain was observed. Post-synaptic density protein 95 (PSD-95) is a synaptic protein found only in the brain. Drosophila disc large tumor suppressor (Dlg1) and zona occludens 1 (ZO-1) both play an important role at cell junctions and in cell signaling complexes. Since the discovery of PDZ domains more than 20 years ago, hundreds of additional PDZ domains have been identified. The first published use of the phrase “PDZ domain” was not in a paper, but a letter. In September 1995, Dr.Mary B. Kennedy
Mary Bernadette Kennedy (born 1947) is an American biochemist and neuroscientist. She is a member of the American Academy of Arts and Sciences, and is the Allen and Lenabelle Davis Professor of Biology at the California Institute of Technology, w ...
of the California Institute of Technology wrote a letter of correction to Trends in Biomedical Sciences. Earlier that year, another set of scientists had claimed to discover a new protein domain which they called a DHR domain. Dr. Kennedy refuted that her lab had previously described exactly the same domain as a series of “GLGF repeats”. She continued to explain that in order to “better reflect the origin and distribution of the domain,” the new title of the domain would be changed. Thus, the name “PDZ domain” was introduced to the world.
Structure
 PDZ domain structure is partially conserved across the various proteins that contain them. They usually have 5-6 β-strands and one short and one long α-helix. Apart from this conserved fold, the
PDZ domain structure is partially conserved across the various proteins that contain them. They usually have 5-6 β-strands and one short and one long α-helix. Apart from this conserved fold, the secondary structure
Protein secondary structure is the three dimensional form of ''local segments'' of proteins. The two most common secondary structural elements are alpha helices and beta sheets, though beta turns and omega loops occur as well. Secondary struct ...
differs across PDZ domains. This domain tends to be globular with a diameter of about 35 Å.
When studied, PDZ domains are usually isolated as monomers, however some PDZ proteins form dimers. The function of PDZ dimers as compared to monomers is not yet known.
A commonly accepted theory for the binding pocket of the PDZ domain is that it is constituted by several hydrophobic amino acids, apart from the GLGF sequence mentioned earlier, the mainchain atoms of which form a nest (protein structural motif) binding the C-terminal carboxylate of the protein or peptide ligand. Most PDZ domains have such a binding site located between one of the β-strands and the long α-helix.
Functions
PDZ domains have two main functions: Localizing cellular elements, and regulating cellular pathways. The first discovered function of the PDZ domains was to anchor receptor proteins in the membrane to cytoskeletal components. PDZ domains also have regulatory functions on different signaling pathways. Any protein may have one or several PDZ domains, which can be identical or unique (see figure to right). This variety allows these proteins to be very versatile in their interactions. Different PDZ domains in the same protein can have different roles, each binding a different part of the target protein or a different protein altogether.
The first discovered function of the PDZ domains was to anchor receptor proteins in the membrane to cytoskeletal components. PDZ domains also have regulatory functions on different signaling pathways. Any protein may have one or several PDZ domains, which can be identical or unique (see figure to right). This variety allows these proteins to be very versatile in their interactions. Different PDZ domains in the same protein can have different roles, each binding a different part of the target protein or a different protein altogether.
Localization
PDZ domains play a vital role in organizing and maintaining complex scaffolding formations. PDZ domains are found in diverse proteins, but all assist in localization of cellular elements. PDZ domains are primarily involved in anchoring receptor proteins to thecytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is comp ...
. For cells to function properly it is important for components—proteins and other molecules— to be in the right place at the right time. Proteins with PDZ domains bind different components to ensure correct arrangements. In the neuron
A neuron, neurone, or nerve cell is an membrane potential#Cell excitability, electrically excitable cell (biology), cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous ...
, making sense of neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neur ...
activity requires specific receptors to be located in the lipid membrane at the synapse. PDZ domains are crucial to this receptor localization process. Proteins with PDZ domains generally associate with both the C-terminus of the receptor and cytoskeletal elements in order to anchor the receptor to the cytoskeleton and keep it in place. Without such an interaction, receptors would diffuse out of the synapse due to the fluid nature of the lipid membrane.
PDZ domains are also utilized to localize elements other than receptor proteins. In the human brain, nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
often acts in the synapse to modify cGMP CGMP is an initialism. It can refer to:
*cyclic guanosine monophosphate (cGMP)
*current good manufacturing practice (cGMP)
*CGMP, Cisco Group Management Protocol, the Cisco version of Internet Group Management Protocol
The Internet Group Managem ...
levels in response to NMDA receptor activation. In order to ensure a favorable spatial arrangements, neuronal nitric oxide synthase (nNOS) is brought close to NMDA receptors via interactions with PDZ domains on PSD-95, which concurrently binds nNOS and NMDA receptors. With nNOS located closely to NMDA receptors, it will be activated immediately after calcium ions begin entering the cell.
Regulation
PDZ domains are directly involved in the regulation of different cellular pathways. This mechanism of this regulation varies as PDZ domains are able to interact with a range of cellular components. This regulation is usually a result of the co-localization of multiple signaling molecules such as in the example with nNos and NMDA receptors. Some examples of signaling pathway regulation executed by the PDZ domain include phosphataseenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
activity, mechanosensory signaling, and the sorting pathway of endocytosed receptor proteins.
The signaling pathway of the human protein tyrosine phosphatase non-receptor type 4 (PTPN4) is regulated by PDZ domains. This protein is involved in regulating cell death
Cell death is the event of a biological cell ceasing to carry out its functions. This may be the result of the natural process of old cells dying and being replaced by new ones, as in programmed cell death, or may result from factors such as di ...
. Normally the PDZ domain of this enzyme is unbound. In this unbound state the enzyme is active and prevents cell signaling for apoptosis. Binding the PDZ domain of this phosphatase results in a loss of enzyme activity, which leads to apoptosis. The normal regulation of this enzyme prevents cells from prematurely going through apoptosis. When the regulation of the PTPN4 enzyme is lost, there is increased oncogenic activity as the cells are able to proliferate.
PDZ domains also have a regulatory role in mechanosensory signaling in proprioceptors and vestibular and auditory hair cell
Hair cells are the sensory receptors of both the auditory system and the vestibular system in the ears of all vertebrates, and in the lateral line organ of fishes. Through mechanotransduction, hair cells detect movement in their environment ...
s. The protein Whirlin (WHRN) localizes in the post-synaptic neurons of hair cells that transform mechanical movement into action potential
An action potential occurs when the membrane potential of a specific cell location rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, ...
s that the body can interpret. WHRN proteins contains three PDZ domains. The domains located near the N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
bind to receptor proteins and other signaling components. When the one of these PDZ domains is inhibited, the signaling pathways of the neurons are disrupted, resulting in auditory, visual, and vestibular impairment. This regulation is thought to be based on the physical positioning WHRN and the selectivity of its PDZ domain.
Regulation of receptor proteins occurs when the PDZ domain on the EBP50 protein binds to the C-terminus of the beta-2 adrenergic receptor (β2-AR). EBP50 also associates with a complex that connects to actin
Actin is a protein family, family of Globular protein, globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in myofibril, muscle fibrils. It is found in essentially all Eukaryote, eukaryotic cel ...
, thus serving as a link between the cytoskeleton and β2-AR. The β2-AR receptor is eventually endocytosed, where it will either be consigned to a lysosome for degradation or recycled back to the cell membrane. Scientists have demonstrated that when the Ser-411 residue of the β2-AR PDZ binding domain, which interacts directly with EBP50, is phosphorylated, the receptor is degraded. If Ser-411 is left unmodified, the receptor is recycled. The role played by PDZ domains and their binding sites indicate a regulative relevance beyond simply receptor protein localization.
PDZ domains are being studied further to better understand the role they play in different signaling pathways. Research has increased as these domains have been linked to different diseases including cancer as discussed above.
Regulation of PDZ domain activity
PDZ domain function can be both inhibited and activated by various mechanisms. Two of the most prevalent include allosteric interactions and posttranslational modifications.Post-translational modifications
The most common post-traslational modification seen on PDZ domains isphosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, ...
. This modification is primarily an inhibitor
Inhibitor or inhibition may refer to:
In biology
* Enzyme inhibitor, a substance that binds to an enzyme and decreases the enzyme's activity
* Reuptake inhibitor, a substance that increases neurotransmission by blocking the reuptake of a neurotra ...
of PDZ domain and ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
activity. In some examples, phosphorylation of amino acid side chains eliminates the ability of the PDZ domain to form hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
s, disrupting the normal binding patterns. The end result is a loss of PDZ domain function and further signaling. Another way phosphorylation can disrupt regular PDZ domain function is by altering the charge ratio and further affecting binding and signaling. In rare cases researchers have seen post-translational modifications activate PDZ domain activity but these cases are few.
cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, s ...
s and are susceptible to disulfide bond formation in oxidizing conditions. This modification acts primarily as an inhibitor of PDZ domain function.
Allosteric Interactions
Protein-protein interactions have been observed to alter the effectiveness of PDZ domains binding to ligands. These studies show that allosteric effects of certain proteins can affect the binding affinity for different substrates. Different PDZ domains can even have this allosteric effect on each other. This PDZ-PDZ interaction only acts as an inhibitor. Other experiments have shown that certainenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
s can enhance the binding of PDZ domains. Researchers found that the protein ezrin enhances the binding of the PDZ protein NHERF1.
PDZ proteins
PDZ proteins are a family of proteins that contain the PDZ domain. This sequence of amino-acids is found in many thousands of known proteins. PDZ domain proteins are widespread ineukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bact ...
s and eubacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
, whereas there are very few examples of the protein in archaea. PDZ domains are often associated with other protein domains and these combinations allow them to carry out their specific functions. Three of the most well documented PDZ proteins are PSD-95, GRIP
Grip(s) or The Grip may refer to:
Common uses
* Grip (job), a job in the film industry
* Grip strength, a measure of hand strength
Music
* Grip (percussion), a method for holding a drum stick or mallet
* ''The Grip'', a 1977 album by Arthur Bl ...
, and HOMER
Homer (; grc, Ὅμηρος , ''Hómēros'') (born ) was a Greek poet who is credited as the author of the '' Iliad'' and the '' Odyssey'', two epic poems that are foundational works of ancient Greek literature. Homer is considered one of ...
.
 PSD-95 is a brain synaptic protein with three PDZ domains, each with unique properties and structures that allow PSD-95 to function in many ways. In general, the first two PDZ domains interact with receptors and the third interacts with cytoskeleton-related proteins. The main receptors associated with PSD-95 are NMDA receptors. The first two PDZ domains of PSD-95 bind to the C-terminus of NMDA receptors and anchor them in the membrane at the point of neurotransmitter release. The first two PDZ domains can also interact in a similar fashion with Shaker-type K+ channels. A PDZ interaction between PSD-95,
PSD-95 is a brain synaptic protein with three PDZ domains, each with unique properties and structures that allow PSD-95 to function in many ways. In general, the first two PDZ domains interact with receptors and the third interacts with cytoskeleton-related proteins. The main receptors associated with PSD-95 are NMDA receptors. The first two PDZ domains of PSD-95 bind to the C-terminus of NMDA receptors and anchor them in the membrane at the point of neurotransmitter release. The first two PDZ domains can also interact in a similar fashion with Shaker-type K+ channels. A PDZ interaction between PSD-95, nNOS
Nitric oxide synthase 1 (neuronal), also known as NOS1, is an enzyme that in humans is encoded by the ''NOS1'' gene.
Function
Nitric oxide synthases () (NOSs) are a family of synthases that catalyze the production of nitric oxide (NO) from ...
and syntrophin is mediated by the second PDZ domain. The third and final PDZ domain links to cysteine-rich PDZ-binding protein ( CRIPT), which allows PSD-95 to associate with the cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is comp ...
.
Glutamate receptor interacting protein (GRIP) is a post-synaptic protein that interacts with AMPA receptor
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, AMPAR, or quisqualate receptor) is an ionotropic transmembrane receptor for glutamate (iGluR) that mediates fast synaptic transmission in the ce ...
s in a fashion analogous to PSD-95 interactions with NMDA receptors. When researchers noticed apparent structural homology between the C-termini of AMPA receptors and NMDA receptors, they attempted to determine if a similar PDZ interaction was occurring. A yeast two-hybrid system helped them discover that out of GRIP's seven PDZ domains, two (domains four and five) were essential for binding of GRIP to the AMPA subunit called GluR2. This interaction is vital for proper localization of AMPA receptors, which play a large part in memory storage. Other researchers discovered that domains six and seven of GRIP are responsible for connecting GRIP to a family of receptor tyrosine kinases called ephrin receptors, which are important signaling proteins. A clinical study concluded that Fraser syndrome, an autosomal recessive syndrome that can cause severe deformations, can be caused by a simple mutation in GRIP.
HOMER
Homer (; grc, Ὅμηρος , ''Hómēros'') (born ) was a Greek poet who is credited as the author of the '' Iliad'' and the '' Odyssey'', two epic poems that are foundational works of ancient Greek literature. Homer is considered one of ...
differs significantly from many known PDZ proteins, including GRIP and PSD-95. Instead of mediating receptors near ion channels, as is the case with GRIP and PSD-95, HOMER is involved in metabotropic glutamate signaling. Another unique aspect of HOMER is that it only contains a single PDZ domain, which mediates interactions between HOMER and type 5 metabotropic glutamate receptor ( mGluR5). The single GLGF repeat on HOMER binds amino acids on the C-terminus of mGluR5. HOMER expression is measured at high levels during embryologic stages in rats, suggesting an important developmental function.
Human PDZ proteins
There are roughly 260 PDZ domains in humans. Several proteins contain multiple PDZ domains, so the number of unique PDZ-containing proteins is closer to 180. In the table below are some of the better studied members of this family: The table below contains all known PDZ proteins in humans (alphabetical): There is currently one known virus containing PDZ domains:References
Further reading
* *External links
* * *The PDZ Domain as a Complex Adaptive System
A concise technical summary and a statement of principal findings and ramifications of the PDZ Domain as a Complex Adaptive System
NCBI conserved domains entry
https://www.pdznet.eu
- An EU project to advance our understanding of the cellular signaling pathways and therapeutic potential of proteins comprising PDZ domains in healthy and pathological conditions such as cancer and neurological diseases. {{Protein domains Protein domains Membrane proteins