Oxocarbenium on:
[Wikipedia]
[Google]
[Amazon]
 In
In
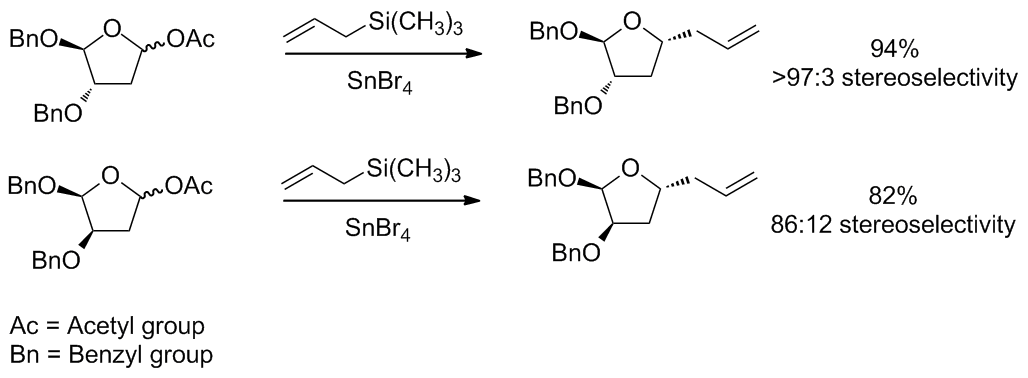
 The
The 
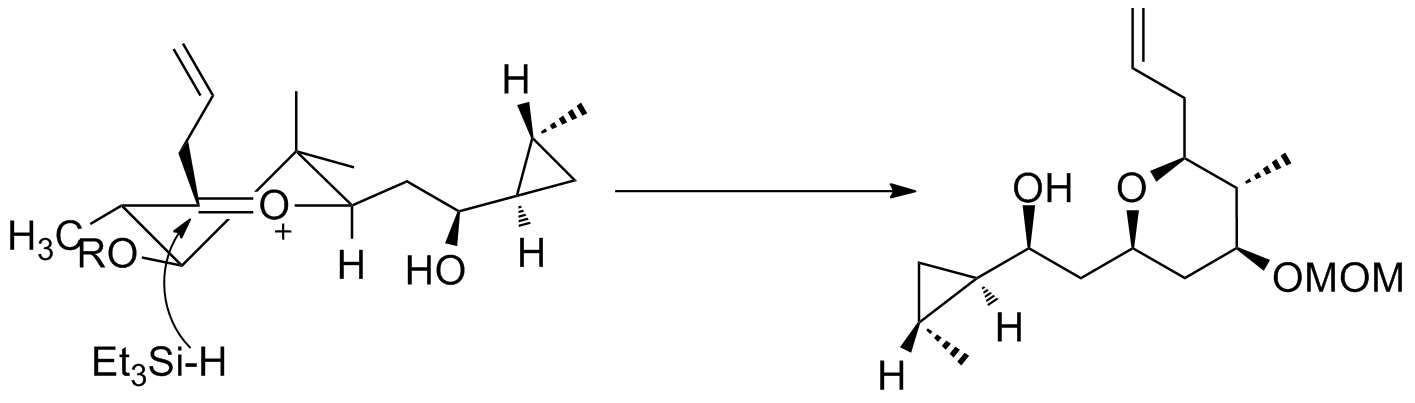 A second example is seen in the key step of the synthesis of (−)-neopeltolide, which uses another six-membered oxocarbenium ring reduction for a diastereoselective hydride addition.
A second example is seen in the key step of the synthesis of (−)-neopeltolide, which uses another six-membered oxocarbenium ring reduction for a diastereoselective hydride addition.
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, an oxocarbenium ion (alternatively spelled oxacarbenium) is a chemical species
Chemical species are a specific form of chemical substance or chemically identical molecular entities that have the same molecular energy level at a specified timescale. These entities are classified through bonding types and relative abundance of ...
characterized by a central sp2-hybridized atom of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, a substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
atom of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, and an overall positive charge that is delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
between the central carbon and oxygen atoms (). An oxocarbenium ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
is represented by two limiting resonance structure
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
s, one in the form of a carbenium ion
The carbenium ion is a kind of cation, positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, whic ...
with the positive charge on carbon () and the other in the form of an oxonium species with the formal charge
In chemistry, a formal charge (F.C. or ), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of rela ...
on oxygen (). As a resonance hybrid, the true structure falls between the two.
Compared to neutral carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
() compounds like ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s () or esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
, the carbenium ion form is a larger contributor to the structure. They are common reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s in the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of glycosidic bond
A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal group o ...
s, and are a commonly used strategy for chemical glycosylation. These ions have since been proposed as reactive intermediates in a wide range of chemical transformations, and have been utilized in the total synthesis of several natural products. In addition, they commonly appear in mechanisms of enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
-catalyzed biosynthesis and hydrolysis of carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s in nature. Anthocyanin
Anthocyanins (), also called anthocyans, are solubility, water-soluble vacuole, vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart named a chemical compou ...
s are natural flavylium dyes, which are stabilized oxocarbenium compounds. Anthocyanins are responsible for the colors of a wide variety of common flowers such as pansies and edible plants such as eggplant
Eggplant (American English, US, Canadian English, CA, Australian English, AU, Philippine English, PH), aubergine (British English, UK, Hiberno English, IE, New Zealand English, NZ), brinjal (Indian English, IN, Singapore English, SG, Malays ...
and blueberry
Blueberries are a widely distributed and widespread group of perennial flowering plants with blue or purple berries. They are classified in the section ''Cyanococcus'' with the genus ''Vaccinium''. Commercial blueberries—both wild (lowbush) ...
.
Electron distribution and reactivity
The best Lewis structure for an oxocarbenium ion contains an oxygen–carbondouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
, with the oxygen atom attached to an additional group and consequently taking on a formal positive charge. In the language of canonical structures (or "resonance"), the polarization of the π bond is described by a secondary carbocationic resonance form, with a formal positive charge on carbon (see above). In terms of frontier molecular orbital theory
In chemistry, frontier molecular orbital theory is an application of molecular orbital theory describing HOMO and LUMO, HOMO–LUMO interactions.
History
In 1952, Kenichi Fukui published a paper in the ''Journal of Chemical Physics'' titled "A m ...
, the Lowest Unoccupied Molecular Orbital (LUMO) of the oxocarbenium ion is a π* orbital that has the large lobe on the carbon atom; the more electronegative oxygen contributes less to the LUMO. Consequently, in an event of a nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
, the carbon is the electrophilic site. Compared to a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
, the polarization of an oxocarbenium ion is accentuated: they more strongly resemble a "true" carbocation, and they are more reactive toward nucleophiles. In organic reactions, ketones are commonly activated by the coordination of a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
or Brønsted acid to the oxygen to generate an oxocarbenium ion as an intermediate.
Numerically, a typical partial charge (derived from Hartree-Fock computations) for the carbonyl carbon of a ketone (like acetone) is ''δ+'' = 0.51. With the addition of an acidic hydrogen to the oxygen atom to produce , the partial charge increases to ''δ+'' = 0.61. In comparison, the nitrogen analogues of ketones and oxocarbenium ions, imines () and iminium ions (), respectively, have partial charges of ''δ+'' = 0.33 and ''δ+'' = 0.54, respectively. The order of partial positive charge on the carbonyl carbon is therefore imine < ketone < iminium < oxocarbenium.
This is also the order of electrophilicity for species containing C=X (X = O, NR) bonds. This order is synthetically significant and explains, for example, why reductive amination
Reductive amination (also known as reductive alkylation) is a form of amination that converts a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is a common method to make amine ...
s are often best carried out at pH = 5 to 6 using sodium cyanoborohydride () or sodium triacetoxyborohydride () as a reagent. Bearing an electron-withdrawing group, sodium cyanoborohydride and sodium triacetoxyborohydride are poorer reducing agents than sodium borohydride, and their direct reaction with ketones is generally a slow and inefficient process. However, the iminium ion (but not the imine itself) formed ''in situ'' during a reductive amination reaction is a stronger electrophile than the ketone starting material and will react with the hydride source at a synthetically useful rate. Importantly, the reaction is conducted under mildly acidic conditions that protonate the imine intermediate to a significant extent, forming the iminium ion, while not being strongly acidic enough to protonate the ketone, which would form the even more electrophilic oxocarbenium ion. Thus, the reaction conditions and reagent ensure that amine is formed selectively from iminium reduction, instead of direct reduction of the carbonyl group (or its protonated form) to form an alcohol.
Formation
Formation of oxocarbenium ions can proceed through several different pathways. Most commonly, the oxygen of a ketone will bind to aLewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
, which activates the ketone, making it a more effective electrophile. The Lewis acid can be a wide range of molecules, from a simple hydrogen atom to metal complexes. The remainder of this article will focus on alkyl oxocarbenium ions, however, where the atom added to the oxygen is a carbon. One way that this sort of ion will form is the elimination of a leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
. In carbohydrate chemistry, this leaving group is often an ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
or ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
. An alternative to elimination is direct deprotonation of the molecule to form the ion, however, this can be difficult and require strong bases to achieve.
Applications to synthesis
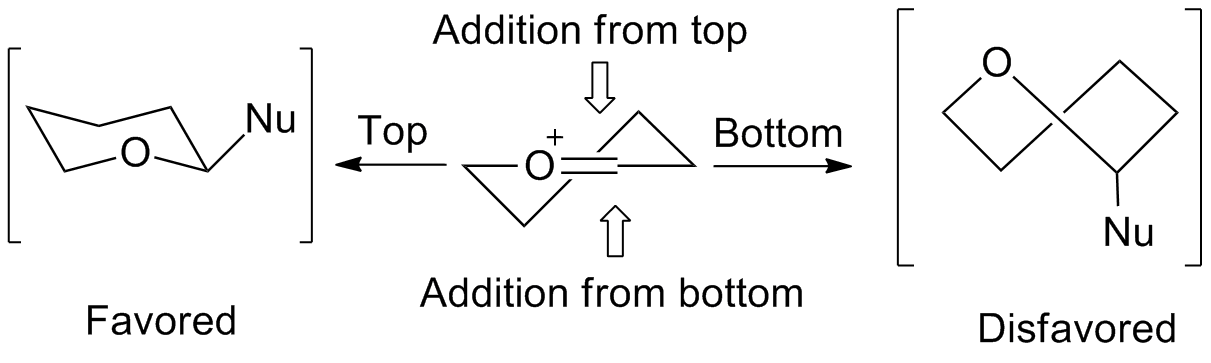
5-membered rings
Thestereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
involved in the reactions of five-membered rings can be predicted by an envelope transition state model. Nucleophiles favor addition from the "inside" of the envelope, or from the top of the figure on the right. The "inside" addition produces a results in a staggered conformation, rather than the eclipsed conformation
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X–A–B–Y is 0°. Such a conformation can exist in any open chain, ...
that results from the "outside" addition.

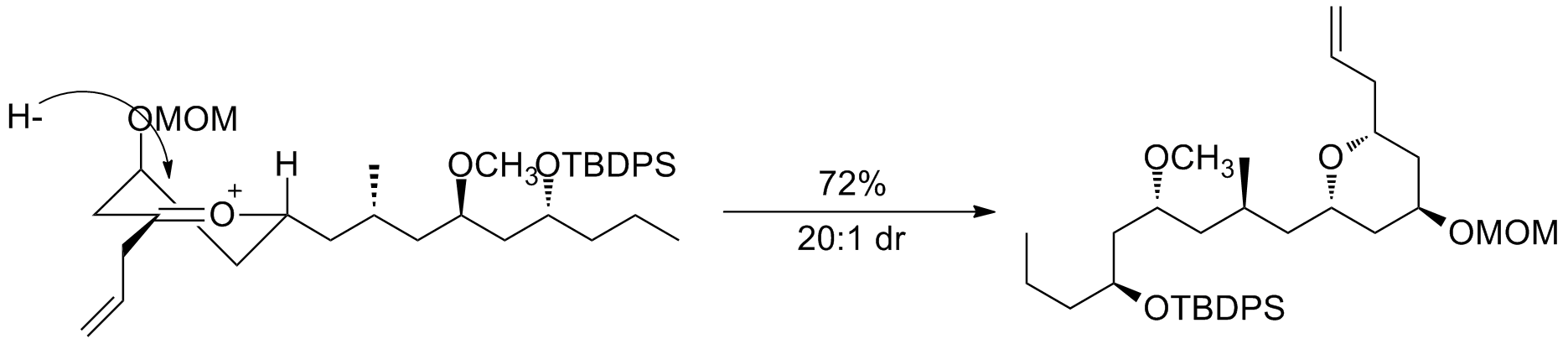
6-membered rings
 The
The transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
model for a six-membered oxocarbenium ring was proposed earlier in 1992 by Woods et al. The general strategy for determining the stereochemistry of a nucleophilic addition to a six-membered ring follows a similar procedure to the case of the five-membered ring. The assumption that one makes for this analysis is that the ring is in the same conformation as cyclohexene
Cyclohexene is a hydrocarbon with the formula . It is a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an chemical intermediate, intermediate in the commercial synthesis of nylon.
Prod ...
, with three carbons and the oxygen in a plane with the two other carbon atome puckered out of the plane, with one above and one below (see the figure to the right). Based on the substituients present on the ring, the lowest energy conformation is determined, keeping in mind steric and stereoelectronic effects (see the section below for a discussion of stereoelectronic effects in oxocarbenium rings). Once this conformation is established, one can consider the nucleophilic addition. The addition will proceed through the low energy chair transition state, rather than the relatively high energy twist-boat. An example of this type of reaction can be seen below. The example also highlights how the stereoelectronic effect exerted by an electronegative substituent flips the lowest energy conformation and leads to opposite selectivity.

Stereoelectronic effects
In analkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
ring that does not contain an oxygen atom, any large substituent prefers to be in an equatorial position, in order to minimize steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
. It has been observed in rings containing oxocarbenium ions that electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
substituents prefer the axial or pseudo-axial positions. When the electronegative atom is in the axial position, its electron density can be donated through space to the positively charged oxygen atom in the ring. This electronic interaction stabilizes the axial conformation. Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
groups, ethers
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ r ...
and halogens
The halogens () are a group (periodic table), group in the periodic table consisting of six chemically related chemical element, elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and ten ...
are examples of substituents that exhibit this phenomenon. Stereoelectronic effect
In chemistry, primarily Organic chemistry, organic and computational chemistry, a stereoelectronic effectAlabugin, I. V. Stereoelectronic Effects: the Bridge between Structure and Reactivity. John Wiley & Sons Ltd, Chichester, UK, 2016. http://eu ...
s must be taken into consideration when determining the lowest energy conformation in the analysis for nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
to an oxocarbenium ion.
Cycloadditions
In organic synthesis, vinyl oxocarbenium ions (structure on right) can be utilized in a wide range ofcycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
reactions. They are commonly employed as dienophiles in the Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexe ...
. An electron withdrawing ketone is often added to the dienophile to increase the rate of the reaction, and these ketones are often converted to vinyl oxocarbenium ions during the reaction. It is not clear that an oxocarbenium ion necessarily will form, but Roush and co-workers demonstrated the oxocarbenium intermediate in the cyclization shown below. Two products were observed in this reaction, which could only form if the oxocarbenium ring is present as an intermediate. +3 +2 +2and +2cycloadditions with oxocarbenium intermediates have also been reported.
Aldol reaction
Chiral oxocarbenium ions have been exploited to carry out highly diastereoselective and enantioselective acetate aldol addition reactions. The oxocarbenium ion is used as anelectrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
in the reaction. When the methyl group increases in size, the diastereoselevtivity increases.
Examples from total synthesis
Oxocarbenium ions have been utilized in total synthesis on several occasions. A major subunit of (+)-clavosolide was synthesized with a reduction of a six-membered oxocarbenium ring. All the large substituents were found in an equatorial position, and the transformation went through the chair transition state, as predicted. A second example is seen in the key step of the synthesis of (−)-neopeltolide, which uses another six-membered oxocarbenium ring reduction for a diastereoselective hydride addition.
A second example is seen in the key step of the synthesis of (−)-neopeltolide, which uses another six-membered oxocarbenium ring reduction for a diastereoselective hydride addition.

Applications to biology
In biological systems, oxocarbenium ions are mostly seen during reactions ofcarbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s. Since sugars are present in the structure of nucleic acids
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic a ...
, with a ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally occurring form, , is a component of the ribonucleotides from which RNA is built, and so this comp ...
sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
present in RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
and a deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. D ...
present in the structure of DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
, their chemistry plays an important role in wide range of cellular functions of nucleic acids. In addition to their functions in nucleotides, sugars are also used for structural components of organisms, as energy storage molecules, cell signaling molecules, protein modification and play key roles in the immune system
The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as Tumor immunology, cancer cells, Parasitic worm, parasitic ...
, fertilization
Fertilisation or fertilization (see American and British English spelling differences#-ise, -ize (-isation, -ization), spelling differences), also known as generative fertilisation, syngamy and impregnation, is the fusion of gametes to give ...
, preventing pathogenesis
In pathology, pathogenesis is the process by which a disease or disorder develops. It can include factors which contribute not only to the onset of the disease or disorder, but also to its progression and maintenance. The word comes .
Descript ...
, blood clotting
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a thrombus, blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of co ...
, and development
Development or developing may refer to:
Arts
*Development (music), the process by which thematic material is reshaped
* Photographic development
*Filmmaking, development phase, including finance and budgeting
* Development hell, when a proje ...
. The abundance of sugar chemistry in biological processes leads many reaction mechanisms to proceed through oxocarbenium ions. Several important biological reactions that utilize oxocarbenium ions are outlined in this section.
Nucleotide biosynthesis
Nucleotides can undergo enzyme-catalyzed intramolecular cyclization in order to produce several important biological molecules. These cyclizations typically proceed through an oxocarbenium intermediate. An example of this reaction can be seen in the cyclization cyclic ADP ribose, which is an important molecule for intracellularcalcium signaling
Calcium signaling is the use of calcium ions (Ca2+) to communicate and drive intracellular processes often as a step in signal transduction. Ca2+ is important for a wide variety of cellular signaling pathways. Once Ca2+ enters the cytosol of the ...
.
Glycosidases
A glycosidase is an enzyme that catalyzes the breakdown of aglycosidic linkage
A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal group o ...
to produce two smaller sugars. This process has important implications in the utilization of stored energy, like glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. It is the main storage form of glucose in the human body.
Glycogen functions as one of three regularly used forms ...
in animals, as well as in the breakdown of cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
by organisms that feed on plants. In general, aspartic or glutamic acid residues in the active site of the enzyme catalyze the hydrolysis of the glycosidic bond. The mechanism of these enzymes involves an oxocarbenium ion intermediate, a general example of which is shown below.
See also
*Carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
* Chemical glycosylation
* Glycosyl donor A glycosyl donor is a carbohydrate mono- or oligosaccharide that will react with a suitable glycosyl acceptor to form a new glycosidic bond. By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new g ...
* Glycosidase
* Oxocarbon anion
References
{{reflist Carbohydrate chemistry Organic reactions Carbocations Oxycations