Orthoformate on:
[Wikipedia]
[Google]
[Amazon]
 In
In

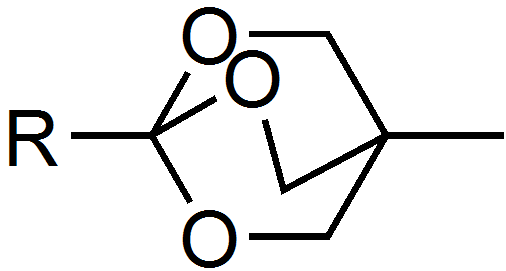 Examples of orthoesters include the reagents
Examples of orthoesters include the reagents
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, an ortho ester is a functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
containing three alkoxy group
In chemistry, the alkoxy group is an alkyl group which is Single bond, singularly bonded to oxygen; thus . Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the ...
s attached to one carbon atom, i.e. with the general formula . Orthoesters may be considered as products of exhaustive alkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
of unstable orthocarboxylic acids and it is from these that the name 'ortho ester' is derived. An example is ethyl orthoacetate
Triethyl orthoacetate is the organic compound with the formula CH3C(OC2H5)3. It is the ethyl orthoester of acetic acid. It is a colorless oily liquid.
Triethyl orthoacetate is used in organic synthesis for acetylation
:
In chemistry, acetylat ...
, , more correctly known as 1,1,1-triethoxyethane.
Synthesis
Ortho esters can be prepared by thePinner reaction
The Pinner reaction refers to the acid catalysed reaction of a nitrile with an alcohol to form an imino ester salt (alkyl imidate salt); this is sometimes referred to as a Pinner salt.
The reaction is named after Adolf Pinner, who first described ...
, in which nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or pr ...
s react with alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s in the presence of one equivalent of hydrogen chloride. The reaction proceeds by formation of imido ester hydrochloride:
:RCN + OH + HCl → C(O)=NH2sup>+Cl−
Upon standing in the presence of excess alcohol, this intermediate converts to the ortho ester:
: C(O)=NH2sup>+Cl− + 2OH → RC(O)3 + NH4Cl
The reaction requires anhydrous conditions, and ideally a nonpolar solvent.
Acid chlorides can also drive the reaction from the corresponding amide, e.g.:
:HCONH2 + BzCl → HC(OBz)NH2Cl
:HC(OBz)NH2Cl + ROH → HC(OR)3 + NH4Cl + BzOH.
Although a less common method, ortho esters were first produced by reaction of 1,1,1-trichloroalkanes with sodium alkoxide:
:RCCl3 + 3NaO → RC(O)3 + 3NaCl
Compounds with an adjacent hydrogen atom on R tend to undergo elimination instead. Traditional ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s can be converted to α,αdichloro ethers with phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula . It is one of the most important phosphorus chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, althoug ...
. The resulting halogenated compounds undergo ether synthesis like the trichloroalkanes.
Carboxylic acids naturally form a trithio ortho ester when heated with a mercaptan
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
of appropriate stoichiometry. The resulting compound undergoes transesterification
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the r ...
to a traditional orthoester in the presence of zinc chloride
Zinc chloride is an Inorganic chemistry, inorganic chemical compound with the chemical formula, formula ZnCl2·''n''H2O, with ''n'' ranging from 0 to 4.5, forming water of hydration, hydrates. Zinc chloride, anhydrous and its hydrates, are colo ...
. Traditional transesterification from a cheaper ortho ester is also possible.
Reactions
Hydrolysis
Ortho esters are readilyhydrolyzed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
in mild aqueous acid to form ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s:
: RC(O)3 + H2O → RCO2 + 2 OH
For example, trimethyl orthoformate
Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH3)3. A colorless liquid, it is the simplest orthoester. It is a reagent used in organic synthesis for the formation of methyl ethers. The product of reaction of an aldeh ...
CH(OCH3)3 may be hydrolyzed (under acidic conditions) to methyl formate
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of a carboxylate ester, it is a colourless liquid with an ethereal odour, high vapor pressure, and low surface tension. The gas form is odourl ...
and methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
; and may be further hydrolyzed (under alkaline conditions) to salts of formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
and methanol.
:
Johnson–Claisen rearrangement
The Johnson–Claisen rearrangement is the reaction of anallyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
ic alcohol with an ortho ester containing a deprotonatable alpha carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
(e.g. triethyl orthoacetate
Triethyl orthoacetate is the organic compound with the formula CH3C(OC2H5)3. It is the ethyl orthoester of acetic acid. It is a colorless oily liquid.
Triethyl orthoacetate is used in organic synthesis for acetylation
:
In chemistry, acetyla ...
) to give a ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
.
:
Bodroux–Chichibabin aldehyde synthesis
In theBodroux–Chichibabin aldehyde synthesis The Bodroux–Chichibabin aldehyde synthesis is a chemical reaction whereby a Grignard reagent is converted to an aldehyde one carbon longer.
:
Reaction of a Grignard reagent with triethyl orthoformate gives an acetal, which can be hydrolyzed
...
an ortho ester reacts with a Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
to form an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
; this is an example of a formylation reaction
Formylation refers to any chemical processes in which a compound is functionalized with a formyl group (-CH=O). In organic chemistry, the term is most commonly used with regards to aromatic compounds (for example the conversion of benzene to benz ...
.
:
Examples
: Hygromycin B, an antibiotic, is one of several naturally occurring ortho esters.">antibiotic.html" ;"title="Hygromycin B, an antibiotic">Hygromycin B, an antibiotic, is one of several naturally occurring ortho esters. Examples of orthoesters include the reagents
Examples of orthoesters include the reagents trimethyl orthoformate
Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH3)3. A colorless liquid, it is the simplest orthoester. It is a reagent used in organic synthesis for the formation of methyl ethers. The product of reaction of an aldeh ...
and triethylorthoacetate. Another example is the bicyclic OBO protecting group (4-methyl-2,6,7-trioxa-bicyclo[2.2.2]octan-1-yl) which is formed by the action of (3-methyloxetan-3-yl)methanol on activated carboxylic acids in the presence of Lewis acids. The group is base stable and can be cleaved in two steps under mild conditions, mildly acidic hydrolysis yields the ester of tris(hydroxymethyl)ethane which is then cleaved using e.g. an aqueous carbonate solution.
The threefold symmetry of the cyclohexanehexol isomer ''scyllo''-inositol (scyllitol) yields the triply-bridged orthoformate esters scyllitol orthoformate with an adamantane
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the mo ...
-like skeleton, and scyllitol bis-orthoformate with two fused adamantane-like skeletons.
Hyo Won Lee and Yoshito Kishi (1985): "Synthesis of mono- and unsymmetrical bis-orthoesters of ''scyllo''-inositol". ''Journal of Organic Chemistry'', volume 50, issue 22, pages 4402–4404
See also
*Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
, C(OR)2R2
* Orthocarbonate, C(OR)4.
References
{{Reflist Functional groups