Organobismuth Compounds on:
[Wikipedia]
[Google]
[Amazon]
Organobismuth chemistry is the
 Triorganobismuth(III) compounds are monomeric with pyramidal structures reminiscent of organophosphorus(III) chemistry. The halides however adopt hypervalent structures. This trend is illustrated by the sheet-like structure adopted by methylbismuth dichloride.
Most aliphatic organobismuth(III) compounds oxidize easily, with the lighter members
Triorganobismuth(III) compounds are monomeric with pyramidal structures reminiscent of organophosphorus(III) chemistry. The halides however adopt hypervalent structures. This trend is illustrated by the sheet-like structure adopted by methylbismuth dichloride.
Most aliphatic organobismuth(III) compounds oxidize easily, with the lighter members
 Likewise acylchlorides react under Pd(0) catalysis to form a variety of phenyl ketones. Although not formally arenes, tricyclopropylbismuth(III) reagents react with aryl halides and triflates under Pd(0) catalysis in a similar fashion to afford a variety of aryl and heteroaryl cyclopropanes:
Likewise acylchlorides react under Pd(0) catalysis to form a variety of phenyl ketones. Although not formally arenes, tricyclopropylbismuth(III) reagents react with aryl halides and triflates under Pd(0) catalysis in a similar fashion to afford a variety of aryl and heteroaryl cyclopropanes:
 In the reaction, the bismuth(V) reagent loses an aryl group and binds to oxygen. The subsequent arylation and elimination are asynchronous and concerted:
In the reaction, the bismuth(V) reagent loses an aryl group and binds to oxygen. The subsequent arylation and elimination are asynchronous and concerted:
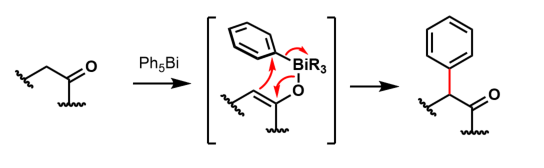 Adjacent electron-pair donors determine regioselectivity.
In the presence of a copper salt, such reagents arylate amines and alcohols. Under
Adjacent electron-pair donors determine regioselectivity.
In the presence of a copper salt, such reagents arylate amines and alcohols. Under
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
of organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, an ...
containing a carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
to bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
. Applications are few. The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi. The first reported use of bismuth in organic chemistry was in oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of alcohols by Frederick Challenger in 1934 (using Ph3Bi(OH)2). Knowledge about methylated species of bismuth in environmental and biological media is limited.
Discovery
Triethylbismuth, the first known organobismuth compound, was prepared in 1850 by Löwig and Schweizer fromiodoethane
Ethyl iodide (also iodoethane) is a colorless flammable chemical compound. It has the chemical formula C2H5I and is prepared by heating ethanol with iodine and phosphorus.''Merck Index of Chemicals and Drugs'', 9th ed., monograph 3753 On contact ...
and a potassium–bismuth alloy. As with most trialkylbismuth compounds, BiEt3 has an extremely pungent and unpleasant odor, and is spontaneously oxidized in air. The chemistry of these complexes first began receiving significant attention when Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s and organolithium compounds
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
became available.
OrganoBi(III) compounds
Properties and structure
pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
. Dialkylhalobismuthines cannot be stored, as they decompose under even inert atmospheres.
Diarylbismuthines are among the most powerful sneezing agents known.
Organobismuth heterocycles are based on Bi(III). The cyclic compound bismole
Bismole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4 H4 BiH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with bismuth replacing the nitrogen atom of pyrrole. Th ...
, a structural analog
A structural analog, also known as a chemical analog or simply an analog, is a chemical compound, compound having a chemical structure, structure similar to that of another compound, but differing from it in respect to a certain component.
It can ...
of pyrrole
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrol ...
, has not been isolated, but substituted bismoles are known. Bismabenzene
Bismabenzene () is the parent representative of a group of organobismuth compounds that are related to benzene with a carbon atom replaced by a bismuth atom. Bismabenzene itself has been synthesised but not isolated because it is too reactive, te ...
has been detected in the laboratory.
Synthesis
The most general and widely-used methodology for homoleptic trialkyl- and triarylbismuth complex synthesis reacts BiX3 withorganolithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
or -magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
reagents:
:BiCl3 + 3RMgX → R3Bi + 3MgXCl
:BiCl3 + 3LiR → BiR3 + 3LiCl.
Triorganobismuth compounds were first prepared instead from K3Bi and organic halides:
:K3Bi + 3RX → BiR3 + 3KX.
This method is generally more difficult and produces a lower yield. However, it remained as of 2006 the only method for e.g., (Me3Si)3Bi synthesis.
Triaryl bismuth(III) compounds are typically air-stable crystalline solids, and the substituents will react before the carbon-bismuth bonds under appropriate conditions:
Asymmetric organobismuth compounds proceed most naturally from the (unstable) organobismuth halides RBiX2 and R2BiX.
Reactions
In industry, triarylbismuth compounds catalyze variousalkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
and alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
polymerizations.
Triarylbismuth compounds have very limited use in organic synthesis. Their bonds are weak, and easily displaced by other elements, metallic or nonmetallic. Such reactions proceed more readily than for the lighter congeners. Of course, triphenylbismuth undergoes redistribution with its trihalide to give the mixed derivatives such as diphenylbismuth chloride (Ph2BiCl). Bbismuth(III) reagents can transfer substituents to thallium(III) compounds: Bi(CH2=CMe)3 + 3 TlCl3 → (CH2=CMe)2TlCl + 2 BiCl3 at −40 °CTriarylbismuth(III) compounds may also be employed in C–N and C–C bond-forming transformations with an appropriate metal co-catalyst. For instance, Barton and coworkers demonstrated that amines could be ''N''-arylated with a bismuth(III) reagent in the presence of copper(II) salt.
 Likewise acylchlorides react under Pd(0) catalysis to form a variety of phenyl ketones. Although not formally arenes, tricyclopropylbismuth(III) reagents react with aryl halides and triflates under Pd(0) catalysis in a similar fashion to afford a variety of aryl and heteroaryl cyclopropanes:
Likewise acylchlorides react under Pd(0) catalysis to form a variety of phenyl ketones. Although not formally arenes, tricyclopropylbismuth(III) reagents react with aryl halides and triflates under Pd(0) catalysis in a similar fashion to afford a variety of aryl and heteroaryl cyclopropanes:
OrganoBi(V) compounds
Structure and stability
The thermal stability of R5M compounds decrease in the order As > Sb > Bi. The aryl compounds are more stable than alkyl compounds. Me5Bi decomposes explosively at 20°C. The nature of the aryl ligands is important in determining whether the complex's geometry is trigonal bipyramidal or square planar and its color. In general, homoleptic compounds of the type Ar5Bi adopt square pyramidal structures. The pentaphenyl compound is deeply colored andthermochromic
Thermochromism is the property of Chemical substance, substances to change color due to a change in temperature. A mood ring is an example of this property used in a consumer product although thermochromism also has more practical uses, such as b ...
, possibly because of equilibration between geometries.
Carboxylates
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,.... ...
rarely form chelating complexes of bismuth. Instead, organobismuth carboxylates are typically polymeric, with each oxygen on the carboxylate coordinating to a different bismuth atom. The same is not true for xanthate
A xanthate is a Salt (chemistry), salt or ester of a xanthic acid. The formula of the salt of xanthic acid is (where R is organyl group and M is usually Sodium, Na or Potassium, K). Xanthate also refers to the anion . The formula of a xanthic a ...
s.
Bismuth halides coordinated to arenes are piano-stool complex
Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples in ...
es.
Synthesis from bismuth(III) compounds
Interestingly, although very few inorganic BiV compounds are known, there is a wide variety of pentacoordinate organobismuth complexes. Triarylorganobismuth complexes easily oxidize to bismuth(V) complexes when treated with chlorine or bromine, giving Ar3BiX2 (X = Cl, Br). Reactions with iodine instead displace ligands to give tricoordinate Ar3−xBiIx, whilst reactions with fluorine are too vigorous for control. All-carbon organobismuth(V) complexes may then be accessed from displacement of the newly formed bismuth-halogen bond with an alkyl or aryl lithium or Grignard reagent. For example: : Me3Bi + SO2Cl2 → Me3BiCl2 + SO2 : Me3BiCl2 + 2 MeLi → Me5Bi + 2 LiCl Unstable, purple Ph5Bi was the first to be synthesized so. Bi(V) easily forms anonium ion
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen (group 15 of the periodic table), chalcogen (group 16), or halogen (group 17). The oldest-known onium ion, and the nam ...
for example by protonation with ''p''-toluenesulfonic acid:
: Ph5Bi + HO3SAr → Ph4Bi+ 3SAr−
Pentaphenylbismuth forms an ate complex
In chemistry, an ate complex is a salt formed by the reaction of a Lewis acid with a Lewis base whereby the central atom (from the Lewis acid) increases its valence and gains a negative formal charge. (In this definition, the meaning of valence ...
upon treatment with phenyl lithium
Phenyllithium is an organometallic agent with the empirical formula . It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses. Crystalline phenyl ...
:
: Ph5Bi + PhLi → Li+ h6Bi−In organic synthesis
Organobismuth(V) reagents are useful for a wide variety of organic transformations. Compared to their lighter congeners, Bi(V) compounds are strong oxidants, dehydrogenating alcohols of all kinds to the carbonyl and cleavingglycols
A diol is a chemical compound containing two hydroxyl groups ( groups). An Aliphatic compound, aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used ...
to the aldehyde. They also engage in aryl transfers
Transfer may refer to:
Arts and media
* ''Transfer'' (2010 film), a German science-fiction movie directed by Damir Lukacevic and starring Zana Marjanović
* ''Transfer'' (1966 film), a short film
* ''Transfer'' (journal), in management studies
* ...
.
The compounds Ph3Bi(OOtBu)2, Ph3BiCO3 and (Ph3BiCl)2O have been investigated for oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
, thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
, phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
, and phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
oxidation. In general, oxidation accelerates when the aryl ligands have electron-withdrawing substituents, and attacks alcohols before thiols or selenides. Hydrazines dehydrogenate to azo compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups).
IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted ...
s and thiols to a mixture of sulfides and disulfides. High yields require strongly basic conditions (absent it, the carbonate is the most effective), suggesting that the active species are triarylbismuth oxides. However, pentaarylbismuth compounds will also abstract hydrogen.
In general, bismuth(V) compounds arylate inefficiently, transferring only one of the five ligands to the substrate and leaving behind a triarylbismuth(III) waste. Reoxidizing the BiIII complex to BiV is hard, and impedes closing a catalytic cycle around this chemistry. Nevertheless, Ph5Bi and Ph3BiCl2 do arylate 1,3-dicarbonyls and arenes:
: Adjacent electron-pair donors determine regioselectivity.
In the presence of a copper salt, such reagents arylate amines and alcohols. Under
Adjacent electron-pair donors determine regioselectivity.
In the presence of a copper salt, such reagents arylate amines and alcohols. Under ultraviolet light
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of th ...
as well, they ''mono''arylate glycols. Steric hindrance governs their high selectivity: secondary alcohols are arylated over tertiary ones, and axial alcohols arylated over equatorial ones.
OrganoBi(I) compounds
Bismuthinidene are analogous tocarbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s since they have the general form R-Bi, with two lone pairs of electrons on the central bismuth(I) atom. Bismuthinidenes are unstable and very reactive due to the unusual +1 oxidation state but can be used in catalysts.
See also
*References
{{ChemicalBondsToCarbon Organobismuth compounds