Nucleotides on:
[Wikipedia]
[Google]
[Amazon]
 Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a
Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a
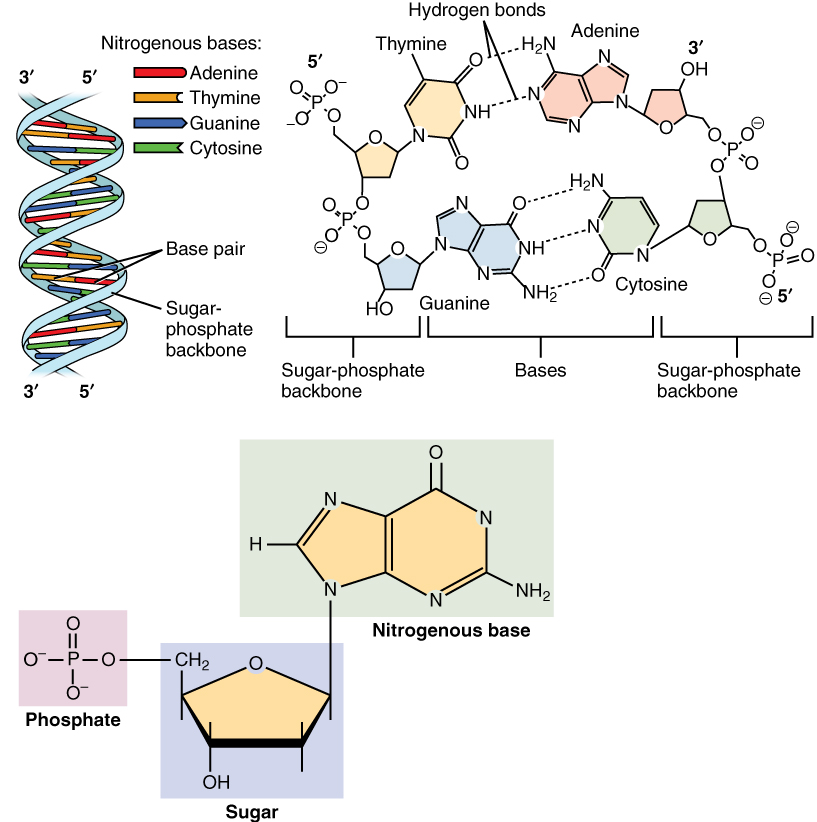 A nucleotide is composed of three distinctive chemical sub-units: a five-carbon sugar molecule, a
A nucleotide is composed of three distinctive chemical sub-units: a five-carbon sugar molecule, a 
 The synthesis of the pyrimidines CTP and UTP occurs in the cytoplasm and starts with the formation of carbamoyl phosphate from
The synthesis of the pyrimidines CTP and UTP occurs in the cytoplasm and starts with the formation of carbamoyl phosphate from
 The de novo synthesis of purine nucleotides by which these precursors are incorporated into the purine ring proceeds by a 10-step pathway to the branch-point intermediate IMP, the nucleotide of the base
The de novo synthesis of purine nucleotides by which these precursors are incorporated into the purine ring proceeds by a 10-step pathway to the branch-point intermediate IMP, the nucleotide of the base
phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
. They serve as monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
ic units of the nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, b ...
– deoxyribonucleic acid
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of a ...
(DNA) and ribonucleic acid
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins ( messenger RNA). RNA and deoxyr ...
(RNA), both of which are essential biomolecules
A biomolecule or biological molecule is loosely defined as a molecule produced by a living organism and essential to one or more typically biological processes. Biomolecules include large macromolecules such as proteins, carbohydrates, lipi ...
within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients
A nutrient is a substance used by an organism to survive, grow and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi and protists. Nutrients can be incorporated into cells for metabolic purposes or excret ...
by the liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
.
Nucleotides are composed of three subunit molecules: a nucleobase
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nuc ...
, a five-carbon sugar (ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally occurring form, , is a component of the ribonucleotides from which RNA is built, and so this comp ...
or deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. D ...
), and a phosphate group consisting of one to three phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
s. The four nucleobases in DNA are guanine
Guanine () (symbol G or Gua) is one of the four main nucleotide bases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine ( uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside ...
, adenine
Adenine (, ) (nucleoside#List of nucleosides and corresponding nucleobases, symbol A or Ade) is a purine nucleotide base that is found in DNA, RNA, and Adenosine triphosphate, ATP. Usually a white crystalline subtance. The shape of adenine is ...
, cytosine
Cytosine () (symbol C or Cyt) is one of the four nucleotide bases found in DNA and RNA, along with adenine, guanine, and thymine ( uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attac ...
, and thymine
Thymine () (symbol T or Thy) is one of the four nucleotide bases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine ...
; in RNA, uracil
Uracil () (nucleoside#List of nucleosides and corresponding nucleobases, symbol U or Ura) is one of the four nucleotide bases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via ...
is used in place of thymine.
Nucleotides also play a central role in metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphate
A nucleoside triphosphate is a nucleoside containing a nitrogenous base bound to a 5-carbon sugar (either ribose or deoxyribose), with three phosphate groups bound to the sugar. They are the molecular precursors of both DNA and RNA, which are chai ...
s, adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known ...
(ATP), guanosine triphosphate
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only di ...
(GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP)—throughout the cell for the many cellular functions that demand energy, including: amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
, protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
and cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
synthesis, moving the cell and cell parts (both internally and intercellularly), cell division, etc..Alberts B, Johnson A, Lewis J, Raff M, Roberts K & Walter P (2002). ''Molecular Biology of the Cell'' (4th ed.). Garland Science. . pp. 120–121. In addition, nucleotides participate in cell signaling
In biology, cell signaling (cell signalling in British English) is the Biological process, process by which a Cell (biology), cell interacts with itself, other cells, and the environment. Cell signaling is a fundamental property of all Cell (biol ...
(cyclic guanosine monophosphate
Cyclic guanosine monophosphate (cGMP) is a cyclic nucleotide derived from guanosine triphosphate (GTP). cGMP acts as a second messenger much like cyclic AMP. Its most likely mechanism of action is activation of intracellular protein kinases in ...
or cGMP and cyclic adenosine monophosphate
Cyclic adenosine monophosphate (cAMP, cyclic AMP, or 3',5'-cyclic adenosine monophosphate) is a second messenger, or cellular signal occurring within cells, that is important in many biological processes. cAMP is a derivative of adenosine tri ...
or cAMP) and are incorporated into important cofactors of enzymatic reactions (e.g., coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric ac ...
, FAD
A fad, trend, or craze is any form of collective behavior that develops within a culture, a generation, or social group in which a group of people enthusiastically follow an impulse for a short time period.
Fads are objects or behaviors tha ...
, FMN, NAD, and NADP+).
In experimental biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
, nucleotides can be radiolabeled using radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
s to yield radionucleotides.
5-nucleotides are also used in flavour enhancers as food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar ( pickling), salt ( salting), smoke ( smoking) and sugar ( crystallization), have been used f ...
to enhance the umami
Umami ( from ), or savoriness, is one of the five basic tastes. It is characteristic of broths and cooked meats.
People taste umami through taste receptors that typically respond to glutamates and nucleotides, which are widely present in me ...
taste, often in the form of a yeast extract.
Structure
 A nucleotide is composed of three distinctive chemical sub-units: a five-carbon sugar molecule, a
A nucleotide is composed of three distinctive chemical sub-units: a five-carbon sugar molecule, a nucleobase
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nuc ...
(the two of which together are called a nucleoside), and one phosphate group
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosp ...
. With all three joined, a nucleotide is also termed a "nucleoside ''mono''phosphate", "nucleoside ''di''phosphate" or "nucleoside ''tri''phosphate", depending on how many phosphates make up the phosphate group.
In nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
s, nucleotides contain either a purine
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted puri ...
or a pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The oth ...
base—i.e., the nucleobase molecule, also known as a nitrogenous base—and are termed ''ribo''nucleotides if the sugar is ribose, or ''deoxyribo''nucleotides if the sugar is deoxyribose. Individual phosphate molecules repetitively connect the sugar-ring molecules in two adjacent nucleotide monomers, thereby connecting the nucleotide monomers of a nucleic acid end-to-end into a long chain. These chain-joins of sugar and phosphate molecules create a 'backbone' strand for a single- or double helix
In molecular biology, the term double helix refers to the structure formed by base pair, double-stranded molecules of nucleic acids such as DNA. The double Helix, helical structure of a nucleic acid complex arises as a consequence of its Nuclei ...
. In any one strand, the chemical orientation ( directionality) of the chain-joins runs from the 5'-end to the 3'-end (''read'': 5 prime-end to 3 prime-end)—referring to the five carbon sites on sugar molecules in adjacent nucleotides. In a double helix, the two strands are oriented in opposite directions, which permits base pairing
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA ...
and complementarity between the base-pairs, all which is essential for replicating or transcribing the encoded information found in DNA.
Nucleic acids then are polymeric macromolecule
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physi ...
s assembled from nucleotides, the monomer-units of nucleic acids. The purine bases adenine
Adenine (, ) (nucleoside#List of nucleosides and corresponding nucleobases, symbol A or Ade) is a purine nucleotide base that is found in DNA, RNA, and Adenosine triphosphate, ATP. Usually a white crystalline subtance. The shape of adenine is ...
and guanine
Guanine () (symbol G or Gua) is one of the four main nucleotide bases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine ( uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside ...
and pyrimidine base cytosine
Cytosine () (symbol C or Cyt) is one of the four nucleotide bases found in DNA and RNA, along with adenine, guanine, and thymine ( uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attac ...
occur in both DNA and RNA, while the pyrimidine bases thymine
Thymine () (symbol T or Thy) is one of the four nucleotide bases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine ...
(in DNA) and uracil
Uracil () (nucleoside#List of nucleosides and corresponding nucleobases, symbol U or Ura) is one of the four nucleotide bases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via ...
(in RNA) occur in just one. Adenine forms a base pair
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA ...
with thymine with two hydrogen bonds, while guanine pairs with cytosine with three hydrogen bonds.
In addition to being building blocks for the construction of nucleic acid polymers, singular nucleotides play roles in cellular energy storage and provision, cellular signaling, as a source of phosphate groups used to modulate the activity of proteins and other signaling molecules, and as enzymatic cofactors, often carrying out redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
reactions. Signaling cyclic nucleotides
A cyclic nucleotide (cNMP) is a single-phosphate nucleotide with a cyclic bond arrangement between the sugar and phosphate groups. Like other nucleotides, cyclic nucleotides are composed of three functional groups: a sugar, a nitrogenous base, a ...
are formed by binding the phosphate group twice to the same sugar molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
, bridging the 5'- and 3'- hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
s of the sugar. Some signaling nucleotides differ from the standard single-phosphate group configuration, in having multiple phosphate groups attached to different positions on the sugar. Nucleotide cofactors include a wider range of chemical groups attached to the sugar via the glycosidic bond
A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal group o ...
, including nicotinamide
Nicotinamide (International nonproprietary name, INN, British Approved Name, BAN ) or niacinamide (United States Adopted Name, USAN ) is a form of vitamin B3, vitamin B3 found in food and used as a dietary supplement and medication. As a suppl ...
and flavin, and in the latter case, the ribose sugar is linear rather than forming the ring seen in other nucleotides.
Synthesis
Nucleotides can be synthesized by a variety of means, bothin vitro
''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in ...
and in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, an ...
.
In vitro, protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
s may be used during laboratory production of nucleotides. A purified nucleoside
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotid ...
is protected to create a phosphoramidite, which can then be used to obtain analogues not found in nature and/or to synthesize an oligonucleotide.
In vivo, nucleotides can be synthesized de novo or recycled through salvage pathways. The components used in de novo nucleotide synthesis are derived from biosynthetic precursors of carbohydrate and amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
metabolism, and from ammonia and carbon dioxide. Recently it has been also demonstrated that cellular bicarbonate metabolism can be regulated by mTORC1 signaling. The liver is the major organ of de novo synthesis of all four nucleotides. De novo synthesis of pyrimidines and purines follows two different pathways. Pyrimidines are synthesized first from aspartate and carbamoyl-phosphate in the cytoplasm to the common precursor ring structure orotic acid, onto which a phosphorylated ribosyl unit is covalently linked. Purines, however, are first synthesized from the sugar template onto which the ring synthesis occurs. For reference, the syntheses of the purine
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted puri ...
and pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The oth ...
nucleotides are carried out by several enzymes in the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
of the cell, not within a specific organelle
In cell biology, an organelle is a specialized subunit, usually within a cell (biology), cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as Organ (anatomy), organs are to th ...
. Nucleotides undergo breakdown such that useful parts can be reused in synthesis reactions to create new nucleotides.
Pyrimidine ribonucleotide synthesis
 The synthesis of the pyrimidines CTP and UTP occurs in the cytoplasm and starts with the formation of carbamoyl phosphate from
The synthesis of the pyrimidines CTP and UTP occurs in the cytoplasm and starts with the formation of carbamoyl phosphate from glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
and CO2. Next, aspartate carbamoyltransferase catalyzes a condensation reaction between aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protein ...
and carbamoyl phosphate to form carbamoyl aspartic acid, which is cyclized into 4,5-dihydroorotic acid by dihydroorotase. The latter is converted to orotate by dihydroorotate oxidase. The net reaction is:
:(''S'')-Dihydroorotate + O2 → Orotate + H2O2
Orotate is covalently linked with a phosphorylated ribosyl unit. The covalent linkage between the ribose and pyrimidine occurs at position C1 of the ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally occurring form, , is a component of the ribonucleotides from which RNA is built, and so this comp ...
unit, which contains a pyrophosphate
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate () and tetrasodium pyrophosphate (), among others. Often pyrophosphates a ...
, and N1 of the pyrimidine ring. Orotate phosphoribosyltransferase (PRPP transferase) catalyzes the net reaction yielding orotidine monophosphate (OMP):
:Orotate + 5-Phospho-α-D-ribose 1-diphosphate (PRPP) → Orotidine 5'-phosphate + Pyrophosphate
Orotidine 5'-monophosphate is decarboxylated by orotidine-5'-phosphate decarboxylase to form uridine monophosphate (UMP). PRPP transferase catalyzes both the ribosylation and decarboxylation reactions, forming UMP from orotic acid in the presence of PRPP. It is from UMP that other pyrimidine nucleotides are derived. UMP is phosphorylated by two kinases to uridine triphosphate (UTP) via two sequential reactions with ATP. First, the diphosphate from UDP is produced, which in turn is phosphorylated to UTP. Both steps are fueled by ATP hydrolysis:
:ATP + UMP → ADP + UDP
:UDP + ATP → UTP + ADP
CTP is subsequently formed by the amination of UTP by the catalytic activity of CTP synthetase. Glutamine is the NH3 donor and the reaction is fueled by ATP hydrolysis, too:
:UTP + Glutamine + ATP + H2O → CTP + ADP + Pi
Cytidine monophosphate (CMP) is derived from cytidine triphosphate (CTP) with subsequent loss of two phosphates.
Purine ribonucleotide synthesis
The atoms that are used to build the purine nucleotides come from a variety of sources:hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids, where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-hydroxypurine. Hyp ...
. AMP and GMP are subsequently synthesized from this intermediate via separate, two-step pathways. Thus, purine moieties are initially formed as part of the ribonucleotides rather than as free bases.
Six enzymes take part in IMP synthesis. Three of them are multifunctional:
* GART (reactions 2, 3, and 5)
* PAICS (reactions 6, and 7)
* ATIC (reactions 9, and 10)
The pathway starts with the formation of PRPP. PRPS1 is the enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
that activates R5P, which is formed primarily by the pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt or HMP shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (five-carbon sugars) as well as ribose 5-ph ...
, to PRPP by reacting it with ATP. The reaction is unusual in that a pyrophosphoryl group is directly transferred from ATP to C1 of R5P and that the product has the α configuration about C1. This reaction is also shared with the pathways for the synthesis of Trp, His, and the pyrimidine nucleotides. Being on a major metabolic crossroad and requiring much energy, this reaction is highly regulated.
In the first reaction unique to purine nucleotide biosynthesis, PPAT catalyzes the displacement of PRPP's pyrophosphate
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate () and tetrasodium pyrophosphate (), among others. Often pyrophosphates a ...
group (PPi) by an amide nitrogen donated from either glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
(N), glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
(N&C), aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protein ...
(N), folic acid
Folate, also known as vitamin B9 and folacin, is one of the B vitamins. Manufactured folic acid, which is converted into folate by the body, is used as a dietary supplement and in food fortification as it is more stable during processing and ...
(C1), or CO2. This is the committed step in purine synthesis. The reaction occurs with the inversion of configuration about ribose C1, thereby forming β- 5-phosphorybosylamine (5-PRA) and establishing the anomeric form of the future nucleotide.
Next, a glycine is incorporated fueled by ATP hydrolysis, and the carboxyl group forms an amine bond to the NH2 previously introduced. A one-carbon unit from folic acid coenzyme N10-formyl-THF is then added to the amino group of the substituted glycine followed by the closure of the imidazole ring. Next, a second NH2 group is transferred from glutamine to the first carbon of the glycine unit. A carboxylation of the second carbon of the glycin unit is concomitantly added. This new carbon is modified by the addition of a third NH2 unit, this time transferred from an aspartate residue. Finally, a second one-carbon unit from formyl-THF is added to the nitrogen group and the ring is covalently closed to form the common purine precursor inosine monophosphate (IMP).
Inosine monophosphate is converted to adenosine monophosphate in two steps. First, GTP hydrolysis fuels the addition of aspartate to IMP by adenylosuccinate synthase, substituting the carbonyl oxygen for a nitrogen and forming the intermediate adenylosuccinate. Fumarate is then cleaved off forming adenosine monophosphate. This step is catalyzed by adenylosuccinate lyase.
Inosine monophosphate is converted to guanosine monophosphate by the oxidation of IMP forming xanthylate, followed by the insertion of an amino group at C2. NAD+ is the electron acceptor in the oxidation reaction. The amide group transfer from glutamine is fueled by ATP hydrolysis.
Pyrimidine and purine degradation
In humans, pyrimidine rings (C, T, U) can be degraded completely to CO2 and NH3 (urea excretion). That having been said, purine rings (G, A) cannot. Instead, they are degraded to the metabolically inerturic acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the Chemical formula, formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the meta ...
which is then excreted from the body. Uric acid is formed when GMP is split into the base guanine and ribose. Guanine is deaminated to xanthine which in turn is oxidized to uric acid. This last reaction is irreversible. Similarly, uric acid can be formed when AMP is deaminated to IMP from which the ribose unit is removed to form hypoxanthine. Hypoxanthine is oxidized to xanthine and finally to uric acid. Instead of uric acid secretion, guanine and IMP can be used for recycling purposes and nucleic acid synthesis in the presence of PRPP and aspartate (NH3 donor).
Prebiotic synthesis of nucleotides
Theories about theorigin of life
Abiogenesis is the natural process by which life arises from abiotic component, non-living matter, such as simple organic compounds. The prevailing scientific hypothesis is that the transition from non-living to organism, living entities on ...
require knowledge of chemical pathways that permit formation of life's key building blocks under plausible prebiotic conditions. The RNA world
The RNA world is a hypothetical stage in the evolutionary history of life on Earth in which self-replicating RNA molecules proliferated before the evolution of DNA and proteins. The term also refers to the hypothesis that posits the existence ...
hypothesis holds that in the primordial soup
Primordial soup, also known as prebiotic soup and Haldane soup, is the hypothetical set of conditions present on the Earth around 3.7 to 4.0 billion years ago. It is an aspect of the heterotrophic theory (also known as the Oparin–Haldane hypothes ...
there existed free-floating ribonucleotides, the fundamental molecules that combine in series to form RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
. Complex molecules like RNA must have arisen from small molecules whose reactivity was governed by physico-chemical processes. RNA is composed of purine
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted puri ...
and pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The oth ...
nucleotides, both of which are necessary for reliable information transfer, and thus Darwinian evolution
Evolution is the change in the heritable Phenotypic trait, characteristics of biological populations over successive generations. It occurs when evolutionary processes such as natural selection and genetic drift act on genetic variation, re ...
. Becker et al. showed how pyrimidine nucleoside
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotid ...
s can be synthesized from small molecules and ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally occurring form, , is a component of the ribonucleotides from which RNA is built, and so this comp ...
, driven solely by wet-dry cycles. Purine nucleosides can be synthesized by a similar pathway. 5'-mono- and di-phosphates also form selectively from phosphate-containing minerals, allowing concurrent formation of polyribonucleotides with both the purine and pyrimidine bases. Thus a reaction network towards the purine and pyrimidine RNA building blocks can be established starting from simple atmospheric or volcanic molecules.
Unnatural base pair (UBP)
An unnatural base pair (UBP) is a designed subunit (ornucleobase
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nuc ...
) of DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
which is created in a laboratory and does not occur in nature. Examples include d5SICS and dNaM. These artificial nucleotides bearing hydrophobic nucleobase
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nuc ...
s, feature two fused aromatic rings that form a (d5SICS–dNaM) complex or base pair in DNA. ''E. coli'' have been induced to replicate a plasmid containing UBPs through multiple generations. This is the first known example of a living organism passing along an expanded genetic code to subsequent generations.
Medical applications of synthetic nucleotides
The applications of synthetic nucleotides vary widely and include disease diagnosis, treatment, or precision medicine. # Antiviral or Antiretroviral agents: several nucleotide derivatives have been used in the treatment against infection withHepatitis
Hepatitis is inflammation of the liver parenchyma, liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), Anorexia (symptom), poor appetite ...
and HIV. Examples of direct nucleoside analog reverse-transcriptase inhibitors ( NRTIs) include Tenofovir disoproxil, Tenofovir alafenamide
Tenofovir alafenamide, sold under the brand name Vemlidy, is an antiviral medication used against hepatitis B and HIV. It is used for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease an ...
, and Sofosbuvir. On the other hand, agents such as Mericitabine, Lamivudine
Lamivudine, commonly called 3TC, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is also used to treat chronic hepatitis B when other options are not possible. It is effective against both HIV-1 and HIV-2. It is typi ...
, Entecavir and Telbivudine must first undergo metabolization via phosphorylation to become activated.
# Antisense oligonucleotides (ASO): synthetic oligonucleotides have been used in the treatment of rare heritable diseases since they can bind specific RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
transcripts and ultimately modulate protein expression. Spinal muscular atrophy, amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND) or—in the United States—Lou Gehrig's disease (LGD), is a rare, Terminal illness, terminal neurodegenerative disease, neurodegenerative disorder that results i ...
, homozygous familial hypercholesterolemia, and primary hyperoxaluria type 1 are all amenable to ASO-based therapy. The application of oligonucleotides is a new frontier in precision medicine and management of conditions which are untreatable.
# Synthetic guide RNA (gRNA): synthetic nucleotides can be used to design gRNA which are essential for the proper function of gene-editing technologies such as CRISPR-Cas9.
Length unit
Nucleotide (abbreviated "nt") is a common unit of length for single-stranded nucleic acids, similar to howbase pair
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA ...
is a unit of length for double-stranded nucleic acids.
Abbreviation codes for degenerate bases
TheIUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
has designated the symbols for nucleotides. Apart from the five (A, G, C, T/U) bases, often degenerate bases are used especially for designing PCR primers. These nucleotide codes are listed here. Some primer sequences may also include the character "I", which codes for the non-standard nucleotide inosine. Inosine occurs in tRNAs and will pair with adenine, cytosine, or thymine. This character does not appear in the following table, however, because it does not represent a degeneracy. While inosine can serve a similar function as the degeneracy "H", it is an actual nucleotide, rather than a representation of a mix of nucleotides that covers each possible pairing needed.
See also
*Biology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and ...
* Chromosome
A chromosome is a package of DNA containing part or all of the genetic material of an organism. In most chromosomes, the very long thin DNA fibers are coated with nucleosome-forming packaging proteins; in eukaryotic cells, the most import ...
* Gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
* Genetics
Genetics is the study of genes, genetic variation, and heredity in organisms.Hartl D, Jones E (2005) It is an important branch in biology because heredity is vital to organisms' evolution. Gregor Mendel, a Moravian Augustinians, Augustinian ...
*
*
*
References
Further reading
* * * * * {{Authority control DNA Molecular biology