Neon Color Circle on:
[Wikipedia]
[Google]
[Amazon]
Neon is a chemical element with the
 Neon was discovered in 1898 by the British chemists Sir William Ramsay (1852–1916) and Morris Travers (1872–1961) in London. Neon was discovered when Ramsay chilled a sample of air until it became a liquid, then warmed the liquid and captured the gases as they boiled off. The gases nitrogen, oxygen, and argon had been identified, but the remaining gases were isolated in roughly their order of abundance, in a six-week period beginning at the end of May 1898. First to be identified was krypton. The next, after krypton had been removed, was a gas which gave a brilliant red light under spectroscopic discharge. This gas, identified in June, was named "neon", the Greek analogue of the Latin ''novum'' ('new') suggested by Ramsay's son. The characteristic brilliant red-orange color emitted by gaseous neon when excited electrically was noted immediately. Travers later wrote: "the blaze of crimson light from the tube told its own story and was a sight to dwell upon and never forget."
A second gas was also reported along with neon, having approximately the same density as argon but with a different spectrum – Ramsay and Travers named it ''metargon''.
However, subsequent spectroscopic analysis revealed it to be argon contaminated with carbon monoxide. Finally, the same team discovered xenon by the same process, in September 1898.
Neon's scarcity precluded its prompt application for lighting along the lines of
Neon was discovered in 1898 by the British chemists Sir William Ramsay (1852–1916) and Morris Travers (1872–1961) in London. Neon was discovered when Ramsay chilled a sample of air until it became a liquid, then warmed the liquid and captured the gases as they boiled off. The gases nitrogen, oxygen, and argon had been identified, but the remaining gases were isolated in roughly their order of abundance, in a six-week period beginning at the end of May 1898. First to be identified was krypton. The next, after krypton had been removed, was a gas which gave a brilliant red light under spectroscopic discharge. This gas, identified in June, was named "neon", the Greek analogue of the Latin ''novum'' ('new') suggested by Ramsay's son. The characteristic brilliant red-orange color emitted by gaseous neon when excited electrically was noted immediately. Travers later wrote: "the blaze of crimson light from the tube told its own story and was a sight to dwell upon and never forget."
A second gas was also reported along with neon, having approximately the same density as argon but with a different spectrum – Ramsay and Travers named it ''metargon''.
However, subsequent spectroscopic analysis revealed it to be argon contaminated with carbon monoxide. Finally, the same team discovered xenon by the same process, in September 1898.
Neon's scarcity precluded its prompt application for lighting along the lines of
 Neon has three stable isotopes: 20Ne (90.48%), 21Ne (0.27%) and 22Ne (9.25%).
21Ne and 22Ne are partly
Neon has three stable isotopes: 20Ne (90.48%), 21Ne (0.27%) and 22Ne (9.25%).
21Ne and 22Ne are partly
at the U.S. Geological Survey, by Eric Caldwell, posted January 2004, retrieved February 10, 2011 In addition, isotopic analysis of exposed terrestrial rocks has demonstrated the cosmogenic (cosmic ray) production of 21Ne. This isotope is generated by
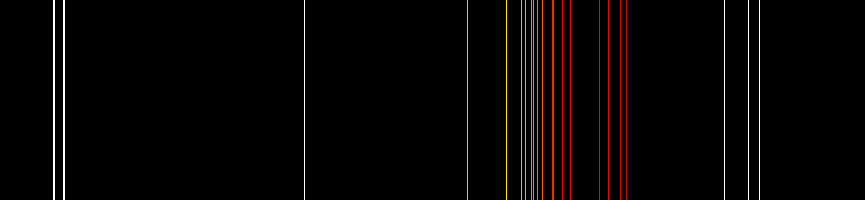 Neon plasma has the most intense light discharge at normal voltages and currents of all the noble gases. The average color of this light to the human eye is red-orange due to many lines in this range; it also contains a strong green line, which is hidden, unless the visual components are dispersed by a spectroscope.
Two quite different kinds of neon lighting are in common use. Neon glow lamps are generally tiny, with most operating between 100 and 250 volts. They have been widely used as power-on indicators and in circuit-testing equipment, but light-emitting diodes (LEDs) now dominate in those applications. These simple neon devices were the forerunners of plasma displays and plasma television screens. Paid access. Neon signs typically operate at much higher voltages (2–15 kilovolts), and the luminous tubes are commonly meters long. The glass tubing is often formed into shapes and letters for signage, as well as architectural and artistic applications.
Neon plasma has the most intense light discharge at normal voltages and currents of all the noble gases. The average color of this light to the human eye is red-orange due to many lines in this range; it also contains a strong green line, which is hidden, unless the visual components are dispersed by a spectroscope.
Two quite different kinds of neon lighting are in common use. Neon glow lamps are generally tiny, with most operating between 100 and 250 volts. They have been widely used as power-on indicators and in circuit-testing equipment, but light-emitting diodes (LEDs) now dominate in those applications. These simple neon devices were the forerunners of plasma displays and plasma television screens. Paid access. Neon signs typically operate at much higher voltages (2–15 kilovolts), and the luminous tubes are commonly meters long. The glass tubing is often formed into shapes and letters for signage, as well as architectural and artistic applications.
 Stable isotopes of neon are produced in stars. Neon's most abundant isotope 20Ne (90.48%) is created by the nuclear fusion of carbon and carbon in the carbon-burning process of
Stable isotopes of neon are produced in stars. Neon's most abundant isotope 20Ne (90.48%) is created by the nuclear fusion of carbon and carbon in the carbon-burning process of
 Neon is the first p-block noble gas, and the first element with a true octet of electrons. It is
Neon is the first p-block noble gas, and the first element with a true octet of electrons. It is
Reuters, 2022-02-25 which may further shift neon production to China.
Neon
at '' The Periodic Table of Videos'' (University of Nottingham)
WebElements.com – Neon
Neon Museum, Las Vegas
{{Authority control Chemical elements Noble gases Coolants Refrigerants Laser gain media Industrial gases
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a atomic electron transition, transition from a high energy state to a lower energy st ...
. The name neon is derived from the Greek word, , neuter singular form of (), meaning 'new'. Neon is chemically inert
Inert may refer to:
* Chemically inert, not chemically reactive
** Inert gas
** Noble gas, historically called inert gas
* Inert knowledge, information which one can express but not use
* Inert waste, waste which is neither chemically nor biol ...
, and no uncharged neon compounds are known. The compounds of neon
Neon compounds are chemical compounds containing the element neon (Ne) with other molecules or elements from the periodic table. Compounds of the noble gas neon were believed not to exist, but there are now known to be molecular ions containing ne ...
currently known include ionic molecules, molecules held together by van der Waals forces and clathrates.
During cosmic nucleogenesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very common element in the universe and solar system (it is fifth in cosmic abundance after hydrogen, helium, oxygen and carbon), it is rare on Earth. It composes about 18.2 ppm of air by volume (this is about the same as the molecular or mole fraction) and a smaller fraction in Earth's crust. The reason for neon's relative scarcity on Earth and the inner (terrestrial) planets is that neon is highly volatile and forms no compounds to fix it to solids. As a result, it escaped from the planetesimals under the warmth of the newly ignited Sun in the early Solar System. Even the outer atmosphere of Jupiter is somewhat depleted of neon, although for a different reason.
Neon gives a distinct reddish-orange glow when used in low- voltage neon glow lamps, high-voltage discharge tubes
A gas-filled tube, also commonly known as a discharge tube or formerly as a Plücker tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric d ...
and neon advertising signs. The red emission line from neon also causes the well known red light of helium–neon lasers. Neon is used in some plasma tube and refrigerant applications but has few other commercial uses. It is commercially extracted by the fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to ...
of liquid air. Since air is the only source, it is considerably more expensive than helium.
History
 Neon was discovered in 1898 by the British chemists Sir William Ramsay (1852–1916) and Morris Travers (1872–1961) in London. Neon was discovered when Ramsay chilled a sample of air until it became a liquid, then warmed the liquid and captured the gases as they boiled off. The gases nitrogen, oxygen, and argon had been identified, but the remaining gases were isolated in roughly their order of abundance, in a six-week period beginning at the end of May 1898. First to be identified was krypton. The next, after krypton had been removed, was a gas which gave a brilliant red light under spectroscopic discharge. This gas, identified in June, was named "neon", the Greek analogue of the Latin ''novum'' ('new') suggested by Ramsay's son. The characteristic brilliant red-orange color emitted by gaseous neon when excited electrically was noted immediately. Travers later wrote: "the blaze of crimson light from the tube told its own story and was a sight to dwell upon and never forget."
A second gas was also reported along with neon, having approximately the same density as argon but with a different spectrum – Ramsay and Travers named it ''metargon''.
However, subsequent spectroscopic analysis revealed it to be argon contaminated with carbon monoxide. Finally, the same team discovered xenon by the same process, in September 1898.
Neon's scarcity precluded its prompt application for lighting along the lines of
Neon was discovered in 1898 by the British chemists Sir William Ramsay (1852–1916) and Morris Travers (1872–1961) in London. Neon was discovered when Ramsay chilled a sample of air until it became a liquid, then warmed the liquid and captured the gases as they boiled off. The gases nitrogen, oxygen, and argon had been identified, but the remaining gases were isolated in roughly their order of abundance, in a six-week period beginning at the end of May 1898. First to be identified was krypton. The next, after krypton had been removed, was a gas which gave a brilliant red light under spectroscopic discharge. This gas, identified in June, was named "neon", the Greek analogue of the Latin ''novum'' ('new') suggested by Ramsay's son. The characteristic brilliant red-orange color emitted by gaseous neon when excited electrically was noted immediately. Travers later wrote: "the blaze of crimson light from the tube told its own story and was a sight to dwell upon and never forget."
A second gas was also reported along with neon, having approximately the same density as argon but with a different spectrum – Ramsay and Travers named it ''metargon''.
However, subsequent spectroscopic analysis revealed it to be argon contaminated with carbon monoxide. Finally, the same team discovered xenon by the same process, in September 1898.
Neon's scarcity precluded its prompt application for lighting along the lines of Moore tube
Daniel McFarlan Moore (February 27, 1869 – June 15, 1936) was a U.S. electrical engineer and inventor. He developed a novel light source, the "Moore lamp", and a business that produced them in the early 1900s. The Moore lamp was the first co ...
s, which used nitrogen and which were commercialized in the early 1900s. After 1902, Georges Claude
Georges Claude (24 September 187023 May 1960) was a French engineer and inventor. He is noted for his early work on the industrial liquefaction of air, for the invention and commercialization of neon lighting, and for a large experiment on genera ...
's company Air Liquide produced industrial quantities of neon as a byproduct of his air-liquefaction business. In December 1910 Claude demonstrated modern neon lighting based on a sealed tube of neon. Claude tried briefly to sell neon tubes for indoor domestic lighting, due to their intensity, but the market failed because homeowners objected to the color. In 1912, Claude's associate began selling neon discharge tubes as eye-catching advertising signs and was instantly more successful. Neon tubes were introduced to the U.S. in 1923 with two large neon signs bought by a Los Angeles Packard car dealership. The glow and arresting red color made neon advertising completely different from the competition. The intense color and vibrancy of neon equated with American society at the time, suggesting a "century of progress" and transforming cities into sensational new environments filled with radiating advertisements and "electro-graphic architecture".
Neon played a role in the basic understanding of the nature of atoms in 1913, when J. J. Thomson, as part of his exploration into the composition of canal rays, channeled streams of neon ions through a magnetic and an electric field and measured the deflection of the streams with a photographic plate. Thomson observed two separate patches of light on the photographic plate (see image), which suggested two different parabolas of deflection. Thomson eventually concluded that some of the atoms in the neon gas were of higher mass than the rest. Though not understood at the time by Thomson, this was the first discovery of isotopes of stable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
atoms. Thomson's device was a crude version of the instrument we now term a mass spectrometer.
Isotopes
primordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before ...
and partly nucleogenic (i.e. made by nuclear reactions of other nuclides with neutrons or other particles in the environment) and their variations in natural abundance are well understood. In contrast, 20Ne (the chief primordial isotope made in stellar nucleosynthesis) is not known to be nucleogenic or radiogenic. The causes of the variation of 20Ne in the Earth have thus been hotly debated.
The principal nuclear reactions generating nucleogenic neon isotopes start from 24Mg and 25Mg, which produce 21Ne and 22Ne respectively, after neutron capture and immediate emission of an alpha particle. The neutrons that produce the reactions are mostly produced by secondary spallation reactions from alpha particles, in turn derived from uranium-series decay chains. The net result yields a trend towards lower 20Ne/22Ne and higher 21Ne/22Ne ratios observed in uranium-rich rocks such as granites.Resources on Isotopes Periodic Table--Neonat the U.S. Geological Survey, by Eric Caldwell, posted January 2004, retrieved February 10, 2011 In addition, isotopic analysis of exposed terrestrial rocks has demonstrated the cosmogenic (cosmic ray) production of 21Ne. This isotope is generated by
spallation
Spallation is a process in which fragments of material (spall) are ejected from a body due to impact or stress. In the context of impact mechanics it describes ejection of material from a target during impact by a projectile. In planetary p ...
reactions on magnesium, sodium, silicon, and aluminium. By analyzing all three isotopes, the cosmogenic component can be resolved from magmatic neon and nucleogenic neon. This suggests that neon will be a useful tool in determining cosmic exposure ages of surface rocks and meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or Natural satellite, moon. When the ...
s.
Neon in solar wind contains a higher proportion of 20Ne than nucleogenic and cosmogenic sources. Neon content observed in samples of volcanic gases and diamonds is also enriched in 20Ne, suggesting a primordial, possibly solar origin.
Characteristics
Neon is the second-lightest noble gas, after helium. It glows reddish-orange in a vacuum discharge tube. It has over 40 times the refrigerating capacity (per unit volume) of liquid helium and three times that of liquid hydrogen. In most applications it is a less expensiverefrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
than helium.
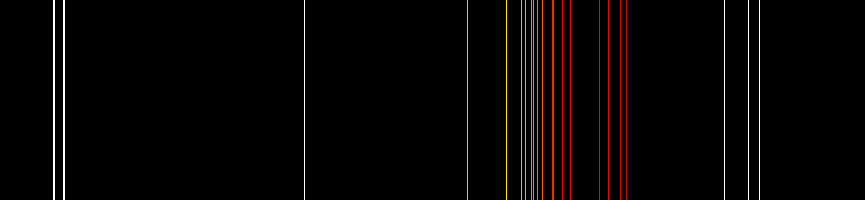 Neon plasma has the most intense light discharge at normal voltages and currents of all the noble gases. The average color of this light to the human eye is red-orange due to many lines in this range; it also contains a strong green line, which is hidden, unless the visual components are dispersed by a spectroscope.
Two quite different kinds of neon lighting are in common use. Neon glow lamps are generally tiny, with most operating between 100 and 250 volts. They have been widely used as power-on indicators and in circuit-testing equipment, but light-emitting diodes (LEDs) now dominate in those applications. These simple neon devices were the forerunners of plasma displays and plasma television screens. Paid access. Neon signs typically operate at much higher voltages (2–15 kilovolts), and the luminous tubes are commonly meters long. The glass tubing is often formed into shapes and letters for signage, as well as architectural and artistic applications.
Neon plasma has the most intense light discharge at normal voltages and currents of all the noble gases. The average color of this light to the human eye is red-orange due to many lines in this range; it also contains a strong green line, which is hidden, unless the visual components are dispersed by a spectroscope.
Two quite different kinds of neon lighting are in common use. Neon glow lamps are generally tiny, with most operating between 100 and 250 volts. They have been widely used as power-on indicators and in circuit-testing equipment, but light-emitting diodes (LEDs) now dominate in those applications. These simple neon devices were the forerunners of plasma displays and plasma television screens. Paid access. Neon signs typically operate at much higher voltages (2–15 kilovolts), and the luminous tubes are commonly meters long. The glass tubing is often formed into shapes and letters for signage, as well as architectural and artistic applications.
Occurrence
 Stable isotopes of neon are produced in stars. Neon's most abundant isotope 20Ne (90.48%) is created by the nuclear fusion of carbon and carbon in the carbon-burning process of
Stable isotopes of neon are produced in stars. Neon's most abundant isotope 20Ne (90.48%) is created by the nuclear fusion of carbon and carbon in the carbon-burning process of stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
. This requires temperatures above 500 megakelvins, which occur in the cores of stars of more than 8 solar masses.
Neon is abundant on a universal scale; it is the fifth most abundant chemical element in the universe by mass, after hydrogen, helium, oxygen, and carbon (see chemical element). Its relative rarity on Earth, like that of helium, is due to its relative lightness, high vapor pressure at very low temperatures, and chemical inertness, all properties which tend to keep it from being trapped in the condensing gas and dust clouds that formed the smaller and warmer solid planets like Earth.
Neon is monatomic, making it lighter than the molecules of diatomic nitrogen and oxygen which form the bulk of Earth's atmosphere; a balloon filled with neon will rise in air, albeit more slowly than a helium balloon.
Neon's abundance in the universe is about 1 part in 750; in the Sun and presumably in the proto-solar system nebula, about 1 part in 600. The Galileo spacecraft
''Galileo'' was an American robotic space probe that studied the planet Jupiter and its moons, as well as the asteroids Gaspra and Ida. Named after the Italian astronomer Galileo Galilei, it consisted of an orbiter and an entry probe. It was ...
atmospheric entry probe found that even in the upper atmosphere of Jupiter, the abundance of neon is reduced (depleted) by about a factor of 10, to a level of 1 part in 6,000 by mass. This may indicate that even the ice- planetesimals, which brought neon into Jupiter from the outer solar system, formed in a region which was too warm to retain the neon atmospheric component (abundances of heavier inert gases on Jupiter are several times that found in the Sun).
Neon comprises 1 part in 55,000 in the Earth's atmosphere, or 18.2 ppm by volume (this is about the same as the molecule or mole fraction), or 1 part in 79,000 of air by mass. It comprises a smaller fraction in the crust. It is industrially produced by cryogenic fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to ...
of liquefied air.
On 17 August 2015, based on studies with the Lunar Atmosphere and Dust Environment Explorer (LADEE) spacecraft, NASA scientists reported the detection of neon in the exosphere
The exosphere ( grc, ἔξω "outside, external, beyond", grc, σφαῖρα "sphere") is a thin, atmosphere-like volume surrounding a planet or natural satellite where molecules are gravitationally bound to that body, but where the densit ...
of the moon.
Chemistry
 Neon is the first p-block noble gas, and the first element with a true octet of electrons. It is
Neon is the first p-block noble gas, and the first element with a true octet of electrons. It is inert
Inert may refer to:
* Chemically inert, not chemically reactive
** Inert gas
** Noble gas, historically called inert gas
* Inert knowledge, information which one can express but not use
* Inert waste, waste which is neither chemically nor biol ...
: as is the case with its lighter analogue, helium, no strongly bound neutral molecules containing neon have been identified. The ions Ar">argon.html" ;"title="eargon">Arsup>+, [Nehydrogen">H">argon">Ar">argon.html" ;"title="eargon">Arsup>+, [Nehydrogen">Hsup>+, and [HeNe]+ have been observed from optical and mass spectrometry, mass spectrometric studies. Solid neon clathrate hydrate was produced from water ice and neon gas at pressures 350–480 MPa and temperatures about −30 °C. Ne atoms are not bonded to water and can freely move through this material. They can be extracted by placing the clathrate into a vacuum chamber for several days, yielding ice XVI, the least dense crystalline form of water.
The familiar Pauling electronegativity scale relies upon chemical bond energies, but such values have obviously not been measured for inert helium and neon. The Allen electronegativity scale, which relies only upon (measurable) atomic energies, identifies neon as the most electronegative element, closely followed by fluorine and helium.
The triple point temperature of neon (24.5561 K) is a defining fixed point in the International Temperature Scale of 1990
The International Temperature Scale of 1990 (ITS-90) is an equipment calibration standard specified by the International Committee of Weights and Measures (CIPM) for making measurements on the Kelvin and Celsius temperature scales. It is an appro ...
.
Production
Neon is produced from air incryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
air-separation plants. A gas-phase mixture mainly of nitrogen, neon, and helium is withdrawn from the main condenser at the top of the high-pressure air-separation column and fed to the bottom of a side column for rectification
Rectification has the following technical meanings:
Mathematics
* Rectification (geometry), truncating a polytope by marking the midpoints of all its edges, and cutting off its vertices at those points
* Rectifiable curve, in mathematics
* Recti ...
of the neon. It can then be further purified from helium.
About 70% of global neon supply is produced in Ukraine as a by-product of steel production in Russia. , the company Iceblick, with plants in Odessa
Odesa (also spelled Odessa) is the third most populous city and municipality in Ukraine and a major seaport and transport hub located in the south-west of the country, on the northwestern shore of the Black Sea. The city is also the administrativ ...
and Moscow, supplies 65 per cent of the world's production of neon, as well as 15% of the krypton and xenon.
2022 shortage
Global neon prices jumped by about 600% after the2014 Russian annexation of Crimea
In February and March 2014, Russia invaded and subsequently annexed the Crimean Peninsula from Ukraine. This event took place in the aftermath of the Revolution of Dignity and is part of the wider Russo-Ukrainian War.
The events in Kyiv th ...
, spurring some chip manufacturers to start shifting away from Russian and Ukrainian suppliers and toward suppliers in China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and ...
. The 2022 Russian invasion of Ukraine
On 24 February 2022, in a major escalation of the Russo-Ukrainian War, which began in 2014. The invasion has resulted in tens of thousands of deaths on both sides. It has caused Europe's largest refugee crisis since World War II. An ...
also shut down two companies in Ukraine: LLC «Cryoin engineering» () and LLC «Inhaz» () located in Odessa
Odesa (also spelled Odessa) is the third most populous city and municipality in Ukraine and a major seaport and transport hub located in the south-west of the country, on the northwestern shore of the Black Sea. The city is also the administrativ ...
and Mariupol respectively; that produced about half of the global supply. The closure was predicted to likely exacerbate COVID-19 chip shortage,Ukraine war flashes neon warning lights for chipsReuters, 2022-02-25 which may further shift neon production to China.
Applications
Neon is often used insigns
Signs may refer to:
* ''Signs'' (2002 film), a 2002 film by M. Night Shyamalan
* ''Signs'' (TV series) (Polish: ''Znaki'') is a 2018 Polish-language television series
* ''Signs'' (journal), a journal of women's studies
*Signs (band), an American ...
and produces an unmistakable bright reddish-orange light. Although tube lights with other colors are often called "neon", they use different noble gases or varied colors of fluorescent lighting.
Neon is used in vacuum tubes, high-voltage indicators, lightning arresters, wavemeter tubes, television tubes, and helium–neon lasers. Liquefied neon is commercially used as a cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
in applications not requiring the lower temperature range attainable with more extreme liquid-helium refrigeration.
Neon, as liquid or gas, is relatively expensive – for small quantities, the price of liquid neon can be more than 55 times that of liquid helium. Driving neon's expense is the rarity of neon, which, unlike helium, can only be obtained in usable quantities by filtering it out of the atmosphere.
Semiconductor industry
gas mixtures that include neon are used to power lasers for EUV lithography.See also
* Expansion ratio * Neon sign * Neon lampReferences
External links
Neon
at '' The Periodic Table of Videos'' (University of Nottingham)
WebElements.com – Neon
Neon Museum, Las Vegas
{{Authority control Chemical elements Noble gases Coolants Refrigerants Laser gain media Industrial gases