Negishi Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Negishi coupling is a widely employed
 The actual mechanism of oxidative addition is unresolved, though there are two likely pathways. One pathway is thought to proceed via an SN2 like mechanism resulting in inverted stereochemistry. The other pathway proceeds via concerted addition and retains stereochemistry.
The actual mechanism of oxidative addition is unresolved, though there are two likely pathways. One pathway is thought to proceed via an SN2 like mechanism resulting in inverted stereochemistry. The other pathway proceeds via concerted addition and retains stereochemistry.
 Though the additions are cis- the Pd(II) complex rapidly isomerizes to the trans- complex.
Though the additions are cis- the Pd(II) complex rapidly isomerizes to the trans- complex.
 Next, the
Next, the  The last step in the catalytic pathway of the Negishi coupling is
The last step in the catalytic pathway of the Negishi coupling is  Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R′ resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow trans-cis isomerization first.
A common
Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R′ resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow trans-cis isomerization first.
A common 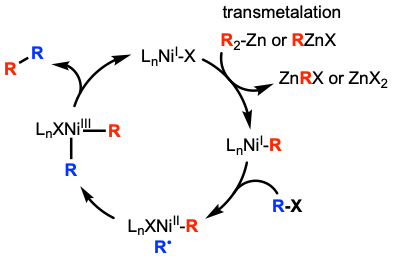 One important factor when contemplating the mechanism of a nickel catalyzed cross coupling is that reductive elimination is facile from NiIII species, but very difficult from NiII species. Kochi and Morrell provided evidence for this by isolating NiII complex Ni(PEt3)2(Me)(''o''-tolyl), which did not undergo reductive elimination quickly enough to be involved in this elementary step.
One important factor when contemplating the mechanism of a nickel catalyzed cross coupling is that reductive elimination is facile from NiIII species, but very difficult from NiII species. Kochi and Morrell provided evidence for this by isolating NiII complex Ni(PEt3)2(Me)(''o''-tolyl), which did not undergo reductive elimination quickly enough to be involved in this elementary step.
 Reactions between sp3-sp3 centers are often more difficult; however, adding an unsaturated ligand with an electron withdrawing group as a cocatalyst improved the yield in some systems. It is believed that added coordination from the unsaturated ligand favors reductive elimination over β-hydride elimination. This also works in some alkyl-aryl systems.
Several asymmetric variants exist and many utilize Pybox ligands.
Reactions between sp3-sp3 centers are often more difficult; however, adding an unsaturated ligand with an electron withdrawing group as a cocatalyst improved the yield in some systems. It is believed that added coordination from the unsaturated ligand favors reductive elimination over β-hydride elimination. This also works in some alkyl-aryl systems.
Several asymmetric variants exist and many utilize Pybox ligands.
 Kibayashi and coworkers utilized the Negishi coupling in the total synthesis of
Kibayashi and coworkers utilized the Negishi coupling in the total synthesis of  δ-trans-tocotrienoloic acid isolated from the plant, Chrysochlamys ulei, is a natural product shown to inhibit
δ-trans-tocotrienoloic acid isolated from the plant, Chrysochlamys ulei, is a natural product shown to inhibit  Smith and Fu demonstrated that their method to couple secondary nucleophiles with secondary alkyl electrophiles could be applied to the formal synthesis of α-cembra-2,7,11-triene-4,6-diol, a target with antitumor activity. They achieved a 61% yield on a gram scale using their method to install an ''iso''-propyl group. This method would be highly adaptable in this application for diversification and installing other alkyl groups to enable structure-activbity relationship (SAR) studies.
Smith and Fu demonstrated that their method to couple secondary nucleophiles with secondary alkyl electrophiles could be applied to the formal synthesis of α-cembra-2,7,11-triene-4,6-diol, a target with antitumor activity. They achieved a 61% yield on a gram scale using their method to install an ''iso''-propyl group. This method would be highly adaptable in this application for diversification and installing other alkyl groups to enable structure-activbity relationship (SAR) studies.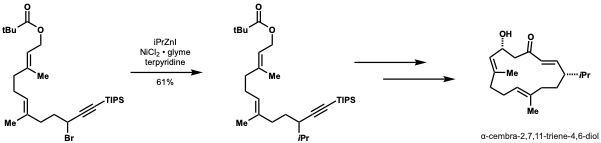 Kirschning and Schmidt applied nickel catalyzed negishi cross-coupling to the first total synthesis of carolacton. In this application, they achieved 82% yield and dr = 10:1.
Kirschning and Schmidt applied nickel catalyzed negishi cross-coupling to the first total synthesis of carolacton. In this application, they achieved 82% yield and dr = 10:1.
 Aryl zincs can be synthesized using mild reaction conditions via a Grignard like intermediate.
:
Organozincs can also be generated in situ and used in a one pot procedure as demonstrated by Knochel et al.
:
Aryl zincs can be synthesized using mild reaction conditions via a Grignard like intermediate.
:
Organozincs can also be generated in situ and used in a one pot procedure as demonstrated by Knochel et al.
:
The Negishi coupling
at www.organic-chemistry.org Carbon-carbon bond forming reactions Condensation reactions Name reactions
transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
catalyzed cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important re ...
. The reaction couples organic halides
Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, orga ...
or triflates
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ...
with organozinc compound
Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.The Chemistry of Organozinc Compounds' (Patai S ...
s, forming carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between on ...
s (C–C) in the process. A palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
(0) species is generally utilized as the catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, though nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.
:* The leaving group X is usually chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
, bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retard ...
, or iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
, but triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
and acetyloxy
In organic chemistry, the acetoxy group (abbr. AcO– or –OAc; IUPAC name: acetyloxy), is a functional group with the formula and the structure . As the ''-oxy'' suffix implies, it differs from the acetyl group () by the presence of an addit ...
groups are feasible as well. X = Cl usually leads to slow reactions.
:* The organic residue R = alkenyl
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
, allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
, alkynyl
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
or propargyl
In organic chemistry, the propargyl group is a functional group of 2- propynyl with the structure . It is an alkyl group derived from propyne ().
The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framework ...
.
:* The halide X′ in the organozinc compound can be chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
, bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
or iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
and the organic residue R′ is alkenyl
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
, allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
, alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
, benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group ().
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substituent ...
, homoallyl, and homopropargyl.
:* The metal M in the catalyst is nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
or palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
:* The ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
L in the catalyst can be triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a l ...
, dppe DPPE may refer to the chemicals:
* 1,2-Bis(diphenylphosphino)ethane
* Dipalmitoylphosphatidylethanolamine
{{disambiguation ...
, BINAP
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This Optical isomerism, chiral diphosphines, diphosphine ligand is widely used in chiral synthesis, asymmetric synthesis. It consists of a pair of 2-diphe ...
, chiraphos or XPhos.
Palladium catalysts in general have higher chemical yield
In chemistry, yield, also known as reaction yield or chemical yield, refers to the amount of product obtained in a chemical reaction. Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis ...
s and higher functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
tolerance.
The Negishi coupling finds common use in the field of total synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
as a method for selectively forming C-C bonds between complex synthetic intermediates. The reaction allows for the coupling of sp3, sp2, and sp carbon atoms, (see orbital hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
) which make it somewhat unusual among the palladium-catalyzed coupling reactions
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important reac ...
. Organozincs are moisture and air sensitive Air sensitivity is a term used, particularly in chemistry, to denote the reactivity of chemical compounds with some constituent of air. Most often, reactions occur with atmospheric oxygen (O2) or water vapor (H2O), although reactions with the other ...
, so the Negishi coupling must be performed in an oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and water free environment, a fact that has hindered its use relative to other cross-coupling reactions that require less robust conditions (i.e. Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
). However, organozincs are more reactive than both organostannanes and organoborates which correlates to faster reaction times.
The reaction is named after Ei-ichi Negishi
was a Japanese chemist who was best known for his discovery of the Negishi coupling. He spent most of his career at Purdue University in the United States, where he was the Herbert C. Brown Distinguished Professor and the director of the Negi ...
who was a co-recipient of the 2010 Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
for the discovery and development of this reaction.
Negishi and coworkers originally investigated the cross-coupling of organoaluminum reagents in 1976 initially employing Ni and Pd as the transition metal catalysts, but noted that Ni resulted in the decay of stereospecifity whereas Pd did not. Transitioning from organoaluminum species to organozinc compounds Negishi and coworkers reported the use of Pd complexes in organozinc coupling reactions and carried out methods studies, eventually developing the reaction conditions into those commonly utilized today. Alongside Richard F. Heck
Richard Frederick Heck (August 15, 1931 – October 9, 2015) was an American chemist noted for the discovery and development of the Heck reaction, which uses palladium to catalyze organic chemical reactions that couple aryl halides with alken ...
and Akira Suzuki
is a Japanese chemist and Nobel Prize Laureate (2010), who first published the Suzuki reaction, the organic reaction of an aryl- or vinyl- boronic acid with an aryl- or vinyl- halide catalyzed by a palladium(0) complex, in 1979.
Early life a ...
, El-ichi Negishi was a co-recipient of the Nobel Prize in Chemistry in 2010, for his work on "palladium-catalyzed cross couplings in organic synthesis".
Reaction mechanism
The reaction mechanism is thought to proceed via a standard Pd catalyzed cross-coupling pathway, starting with a Pd(0) species, which is oxidized to Pd(II) in an oxidative addition step involving the organohalide species. This step proceeds with aryl, vinyl, alkynyl, and acyl halides, acetates, or triflates, with substrates following standardoxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
relative rates (I>OTf>Br≫Cl).
 The actual mechanism of oxidative addition is unresolved, though there are two likely pathways. One pathway is thought to proceed via an SN2 like mechanism resulting in inverted stereochemistry. The other pathway proceeds via concerted addition and retains stereochemistry.
The actual mechanism of oxidative addition is unresolved, though there are two likely pathways. One pathway is thought to proceed via an SN2 like mechanism resulting in inverted stereochemistry. The other pathway proceeds via concerted addition and retains stereochemistry.
 Though the additions are cis- the Pd(II) complex rapidly isomerizes to the trans- complex.
Though the additions are cis- the Pd(II) complex rapidly isomerizes to the trans- complex.
 Next, the
Next, the transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
step occurs where the organozinc reagent exchanges its organic substituent with the halide in the Pd(II) complex, generating the trans- Pd(II) complex and a zinc halide salt. The organozinc substrate can be aryl, vinyl, allyl, benzyl, homoallyl, or homopropargyl. Transmetalation is usually rate limiting
In computer networks, rate limiting is used to control the rate of requests sent or received by a network interface controller. It can be used to prevent DoS attacks and limit web scraping.
Research indicates flooding rates for one zombie machin ...
and a complete mechanistic understanding of this step has not yet been reached though several studies have shed light on this process. Alkylzinc species form higher-order zincate species prior to transmetalation whereas arylzinc species do not. ZnXR and ZnR2 can both be used as reactive reagents, and Zn is known to prefer four coordinate complexes, which means solvent coordinated Zn complexes, such as cannot be ruled out ''a priori''. Studies indicate competing equilibriums exist between cis- and trans- bis alkyl organopalladium complexes, but that the only productive intermediate is the cis complex.
 The last step in the catalytic pathway of the Negishi coupling is
The last step in the catalytic pathway of the Negishi coupling is reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
, which is thought to proceed via a three coordinate transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
, yielding the coupled organic product and regenerating the Pd(0) catalyst. For this step to occur, the aforementioned cis- alkyl organopalladium complex must be formed. Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R′ resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow trans-cis isomerization first.
A common
Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R′ resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow trans-cis isomerization first.
A common side reaction
A side reaction is a chemical reaction that occurs at the same time as the actual main reaction, but to a lesser extent. It leads to the formation of by-product, so that the Yield (chemistry), yield of main product is reduced:
: + B ->[] P1
: + C ...
is homocoupling. In one Negishi model system the formation of homocoupling was found to be the result of a second transmetalation reaction between the diarylmetal intermediate and arylmetal halide:
: Ar–Pd–Ar′ + Ar′–Zn–X → Ar′–Pd–Ar′ + Ar–Zn–X
: Ar′–Pd–Ar′ → Ar′–Ar′ + Pd(0) ''(homocoupling)''
: Ar–Zn–X + H2O → Ar–H + HO–Zn–X ''(reaction accompanied by dehalogenation
In organic chemistry, dehalogenation is a set of chemical reactions that involve the Bond cleavage, cleavage of carbon-halogen bonds; as such, it is the inverse reaction of halogenation. Dehalogenations come in many varieties, including defluorin ...
)''
Nickel catalyzed systems can operate under different mechanisms depending on the coupling partners. Unlike palladium systems which involve only Pd0 or PdII, nickel catalyzed systems can involve nickel of different oxidation states. Both systems are similar in that they involve similar elementary steps: oxidative addition, transmetalation, and reductive elimination. Both systems also have to address issues of β-hydride elimination and difficult oxidative addition of alkyl electrophiles.
For unactivated alkyl electrophiles, one possible mechanism is a transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
first mechanism. In this mechanism, the alkyl zinc species would first transmetalate with the nickel catalyst. Then the nickel would abstract the halide from the alkyl halide resulting in the alkyl radical and oxidation of nickel after addition of the radical.
 One important factor when contemplating the mechanism of a nickel catalyzed cross coupling is that reductive elimination is facile from NiIII species, but very difficult from NiII species. Kochi and Morrell provided evidence for this by isolating NiII complex Ni(PEt3)2(Me)(''o''-tolyl), which did not undergo reductive elimination quickly enough to be involved in this elementary step.
One important factor when contemplating the mechanism of a nickel catalyzed cross coupling is that reductive elimination is facile from NiIII species, but very difficult from NiII species. Kochi and Morrell provided evidence for this by isolating NiII complex Ni(PEt3)2(Me)(''o''-tolyl), which did not undergo reductive elimination quickly enough to be involved in this elementary step.
Scope
The Negishi coupling has been applied the following illustrative syntheses: *unsymmetrical2,2'-bipyridine
The comma is a punctuation mark that appears in several variants in different languages. Some typefaces render it as a small line, slightly curved or straight, but inclined from the vertical; others give it the appearance of a miniature fille ...
s from 2-bromopyridine with tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine palladium) is the chemical compound d(P(C6H5)3)4 often abbreviated Pd( PPh3)4, or rarely PdP4. It is a bright yellow crystalline solid that becomes brown upon d ...
,
*biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
from ''o''-tolylzinc chloride and ''o''-iodotoluene and tetrakis(triphenylphosphine)palladium(0),
*5,7-hexadecadiene from 1-decyne and (''Z'')-1-hexenyl iodide.
Negishi coupling has been applied in the synthesis of hexaferrocenylbenzene:
:
with hexaiodidobenzene, diferrocenylzinc and tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0) or
in d2(dba)3
D2, D02, D.II, D II or D-2 may refer to:
Places
* Dublin 2, a Dublin, Ireland postcode
* Mount Dulang-dulang, the second highest mountain of the Philippines
* D2, a line of Moscow Central Diameters
* D2 Place One and Two, at Cheung Yue Street an ...
is an organopalladium compound. The compound is a complex of palladium(0) with dibenzylideneacetone (dba). It is a dark-purple/brown solid, which is modestly soluble in organic solvents. Bec ...tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
. The yield is only 4% signifying substantial crowding around the aryl core.
In a novel modification palladium is first oxidized by the haloketone ''2-chloro-2-phenylacetophenone'' 1 and the resulting palladium OPdCl complex then accepts both the organozinc compound
Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.The Chemistry of Organozinc Compounds' (Patai S ...
2 and the organotin compound
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered ...
3 in a double transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
:
:
Examples of nickel catalyzed Negishi couplings include sp2-sp2, sp2-sp3, and sp3-sp3 systems. In the system first studied by Negishi, aryl-aryl cross coupling was catalyzed by Ni(PPh3)4 generated ''in situ'' through reduction of Ni(acac)2 with PPh3 and (i-Bu)2AlH.
:
Variations have also been developed to allow for the cross-coupling of aryl and alkenyl partners. In the variation developed by Knochel et al, aryl zinc bromides were reacted with vinyl triflates and vinyl halides.
 Reactions between sp3-sp3 centers are often more difficult; however, adding an unsaturated ligand with an electron withdrawing group as a cocatalyst improved the yield in some systems. It is believed that added coordination from the unsaturated ligand favors reductive elimination over β-hydride elimination. This also works in some alkyl-aryl systems.
Several asymmetric variants exist and many utilize Pybox ligands.
Reactions between sp3-sp3 centers are often more difficult; however, adding an unsaturated ligand with an electron withdrawing group as a cocatalyst improved the yield in some systems. It is believed that added coordination from the unsaturated ligand favors reductive elimination over β-hydride elimination. This also works in some alkyl-aryl systems.
Several asymmetric variants exist and many utilize Pybox ligands.
Industrial applications
The Negishi coupling is not employed as frequently in industrial applications as its cousins theSuzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
and Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after T ...
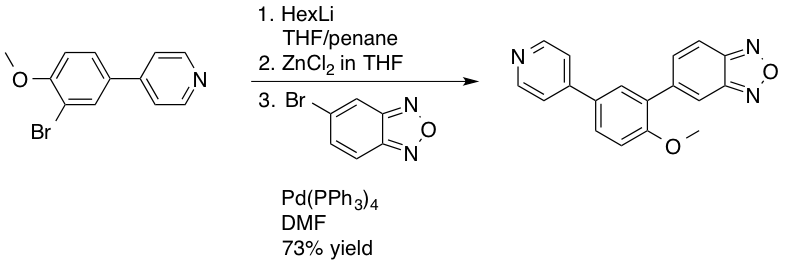
, mostly as a result of the water and air sensitivity of the required aryl or alkyl zinc reagents. In 2003 Novartis
Novartis AG is a Swiss multinational corporation, multinational pharmaceutical company, pharmaceutical corporation based in Basel, Switzerland. Novartis is one of the largest pharmaceutical companies in the world and was the eighth largest by re ...
employed a Negishi coupling in the manufacture of PDE472, a phosphodiesterase
A phosphodiesterase (PDE) is an enzyme that breaks a phosphodiester bond. Usually, ''phosphodiesterase'' refers to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many oth ...
type 4D inhibitor, which was being investigated as a drug lead for the treatment of asthma
Asthma is a common long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wh ...
. The Negishi coupling was used as an alternative to the Suzuki reaction providing improved yields, 73% on a 4.5 kg scale, of the desired benzodioxazole synthetic intermediate.
:
Applications in total synthesis
Where the Negishi coupling is rarely used in industrial chemistry, a result of the aforementioned water and oxygen sensitivity, it finds wide use in the field ofnatural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
s total synthesis. The increased reactivity relative to other cross-coupling reactions makes the Negishi coupling ideal for joining complex intermediates in the synthesis of natural products. Additionally, Zn is more environmentally friendly than other metals such as Sn used in the Stille coupling
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electrop ...
. The Negishi coupling historically is not used as much as the Stille or Suzuki coupling. When it comes to fragment-coupling processes the Negishi coupling is particularly useful, especially when compared to the aforementioned Stille and Suzuki coupling reactions. The major drawback of the Negishi coupling, aside from its water and oxygen sensitivity, is its relative lack of functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
tolerance when compared to other cross-coupling reactions.
(−)-stemoamide is a natural product found in the root extracts of ‘’Stemona tuberosa’’. These extracts have been used Japanese and Chinese folk medicine
Traditional medicine (also known as indigenous medicine or folk medicine) refers to the knowledge, skills, and practices rooted in the cultural beliefs of various societies, especially Indigenous groups, used for maintaining health and treatin ...
to treat respiratory disorders, and (−)-stemoamide is also an anthelminthic. Somfai and coworkers employed a Negishi coupling in their synthesis of (−)-stemoamide. The reaction was implemented mid-synthesis, forming an sp3-sp2 c-c bond between β,γ-unsaturated ester and an intermediate diene 4 with a 78% yield of product 5. Somfai completed the stereoselective total synthesis of (−)-stemoamide in 12-steps with a 20% overall yield.
 Kibayashi and coworkers utilized the Negishi coupling in the total synthesis of
Kibayashi and coworkers utilized the Negishi coupling in the total synthesis of Pumiliotoxin
Pumiliotoxins (PTXs), are one of several toxins found in the skin of poison dart frogs. The frog species, Bibron's toadlet, P. bibronii also produces PTXs to deter predators. Closely related, though more toxic, are allopumiliotoxins, (aPTXs). Other ...
B. Pumiliotoxin B is one of the major toxic alkaloids
Alkaloids are a broad class of naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large variety of organisms i ...
isolated from Dendrobates pumilio, a Panamanian poison frog. These toxic alkaloids display modulatory effects on voltage-dependent sodium channels
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels.
Classification
They are classified into 2 types:
Function
In e ...
, resulting in cardiotonic and myotonic activity. Kibayashi employed the Negishi coupling late stage in the synthesis of Pumiliotoxin B, coupling a homoallylic sp3 carbon on the zinc alkylidene indolizidine 6 with the (E)-vinyl iodide 7 with a 51% yield. The natural product was then obtained after deprotection.
: δ-trans-tocotrienoloic acid isolated from the plant, Chrysochlamys ulei, is a natural product shown to inhibit
δ-trans-tocotrienoloic acid isolated from the plant, Chrysochlamys ulei, is a natural product shown to inhibit DNA polymerase
A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create t ...
β (pol β), which functions to repair DNA via base excision. Inhibition of pol B in conjunction with other chemotherapy
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs (list of chemotherapeutic agents, chemotherapeutic agents or alkylating agents) in a standard chemotherapy re ...
drugs may increase the cytotoxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are toxic metals, toxic chemicals, microbe neurotoxins, radiation particles and even specific neurotransmitters when the system is out of balance. Also some types of d ...
of these chemotherapeutics, leading to lower effective dosages. The Negishi coupling was implemented in the synthesis of δ-trans-tocotrienoloic acid by Hecht and Maloney coupling the sp3 homopropargyl zinc reagent 8 with sp2 vinyl iodide 9. The reaction proceeded with quantitative yield, coupling fragments mid-synthesis en route to the stereoselectively synthesized natural product δ-trans-tocotrienoloic acid.
: Smith and Fu demonstrated that their method to couple secondary nucleophiles with secondary alkyl electrophiles could be applied to the formal synthesis of α-cembra-2,7,11-triene-4,6-diol, a target with antitumor activity. They achieved a 61% yield on a gram scale using their method to install an ''iso''-propyl group. This method would be highly adaptable in this application for diversification and installing other alkyl groups to enable structure-activbity relationship (SAR) studies.
Smith and Fu demonstrated that their method to couple secondary nucleophiles with secondary alkyl electrophiles could be applied to the formal synthesis of α-cembra-2,7,11-triene-4,6-diol, a target with antitumor activity. They achieved a 61% yield on a gram scale using their method to install an ''iso''-propyl group. This method would be highly adaptable in this application for diversification and installing other alkyl groups to enable structure-activbity relationship (SAR) studies. Kirschning and Schmidt applied nickel catalyzed negishi cross-coupling to the first total synthesis of carolacton. In this application, they achieved 82% yield and dr = 10:1.
Kirschning and Schmidt applied nickel catalyzed negishi cross-coupling to the first total synthesis of carolacton. In this application, they achieved 82% yield and dr = 10:1.
Preparation of organozinc precursors
Alkylzinc reagents can be accessed from the corresponding alkyl bromides using iodine in dimethylacetamide (DMAC). The catalytic I2 serves to activate the zinc towards nucleophilic addition. : Aryl zincs can be synthesized using mild reaction conditions via a Grignard like intermediate.
:
Organozincs can also be generated in situ and used in a one pot procedure as demonstrated by Knochel et al.
:
Aryl zincs can be synthesized using mild reaction conditions via a Grignard like intermediate.
:
Organozincs can also be generated in situ and used in a one pot procedure as demonstrated by Knochel et al.
:
Further reading
* *See also
* CPhos *Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after T ...
* Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
References
{{reflist, 32emExternal links
The Negishi coupling
at www.organic-chemistry.org Carbon-carbon bond forming reactions Condensation reactions Name reactions