Morse potential on:
[Wikipedia]
[Google]
[Amazon]
The Morse potential, named after physicist Philip M. Morse, is a convenient
interatomic interaction model for the
 Like the
Like the
potential energy
In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors.
Common types of potential energy include the gravitational potentia ...
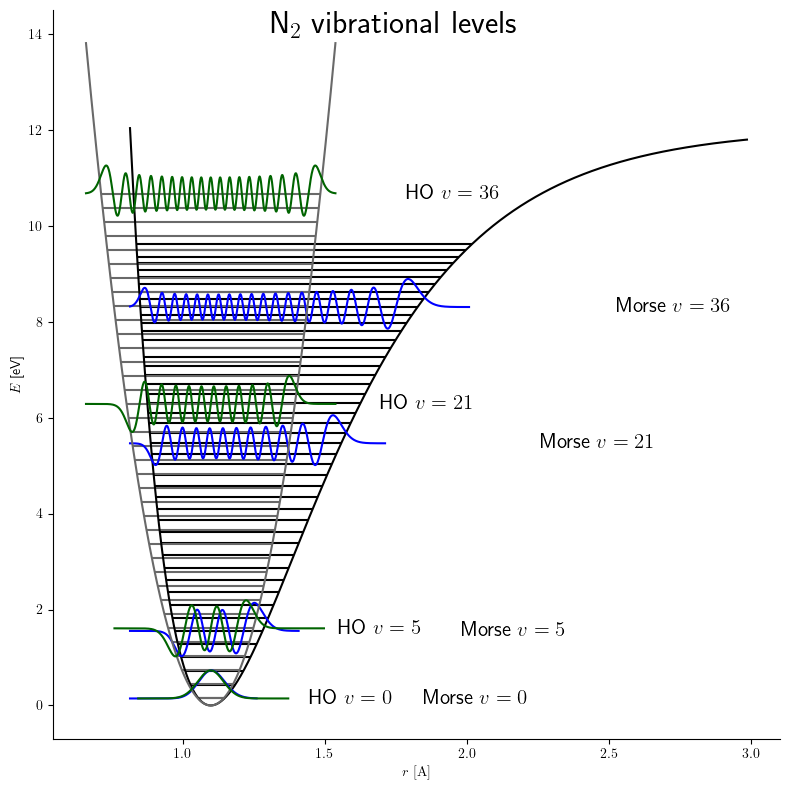
of a diatomic molecule. It is a better approximation for the vibrational structure of the molecule than the quantum harmonic oscillator
量子調和振動子 は、調和振動子, 古典調和振動子 の 量子力学, 量子力学 類似物です。任意の滑らかな ポテンシャル エネルギー, ポテンシャル は通常、安定した 平衡点 の近くで � ...
because it explicitly includes the effects of bond breaking, such as the existence of unbound states. It also accounts for the anharmonicity of real bonds and the non-zero transition probability for overtone and combination bands. The Morse potential can also be used to model other interactions such as the interaction between an atom and a surface. Due to its simplicity (only three fitting parameters), it is not used in modern spectroscopy. However, its mathematical form inspired the MLR ( Morse/Long-range) potential, which is the most popular potential energy function used for fitting spectroscopic data.
Potential energy function
The Morse potential energy function is of the form : Here is the distance between the atoms, is the equilibrium bond distance, is the well depth (defined relative to the dissociated atoms), and controls the 'width' of the potential (the smaller is, the larger the well). The dissociation energy of the bond can be calculated by subtracting the zero point energy from the depth of the well. The force constant (stiffness) of the bond can be found by Taylor expansion of around to the secondderivative
In mathematics, the derivative of a function of a real variable measures the sensitivity to change of the function value (output value) with respect to a change in its argument (input value). Derivatives are a fundamental tool of calculus. ...
of the potential energy function, from which it can be shown that the parameter, , is
:
where is the force constant at the minimum of the well.
Since the zero of potential energy is arbitrary, the equation for the Morse potential can be rewritten any number of ways by adding or subtracting a constant value. When it is used to model the atom-surface interaction, the energy zero can be redefined so that the Morse potential becomes
:
which is usually written as
:
where is now the coordinate perpendicular to the surface. This form approaches zero at infinite and equals at its minimum, i.e. . It clearly shows that the Morse potential is the combination of a short-range repulsion term (the former) and a long-range attractive term (the latter), analogous to the Lennard-Jones potential.
Vibrational states and energies
 Like the
Like the quantum harmonic oscillator
量子調和振動子 は、調和振動子, 古典調和振動子 の 量子力学, 量子力学 類似物です。任意の滑らかな ポテンシャル エネルギー, ポテンシャル は通常、安定した 平衡点 の近くで � ...
, the energies and eigenstates of the Morse potential can be found using operator methods.
One approach involves applying the factorization method
In mathematics, factorization (or factorisation, see English spelling differences) or factoring consists of writing a number or another mathematical object as a product of several ''factors'', usually smaller or simpler objects of the same kin ...
to the Hamiltonian.
To write the stationary states on the Morse potential, i.e. solutions and of the following Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
:
:
it is convenient to introduce the new variables:
:
Then, the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
takes the simple form:
:
:
Its eigenvalue
In linear algebra, an eigenvector () or characteristic vector of a linear transformation is a nonzero vector that changes at most by a scalar factor when that linear transformation is applied to it. The corresponding eigenvalue, often denot ...
s and eigenstates can be written as:
:
where
:
with denoting the largest integer smaller than x.
:
where
and is a generalized Laguerre polynomial:
:
There also exists the following analytical expression for matrix
Matrix most commonly refers to:
* ''The Matrix'' (franchise), an American media franchise
** '' The Matrix'', a 1999 science-fiction action film
** "The Matrix", a fictional setting, a virtual reality environment, within ''The Matrix'' (franchi ...
elements of the coordinate operator:
:
which is valid for and . The eigenenergies in the initial variables have the form:
:
where is the vibrational quantum number and has units of frequency. The latter is mathematically related to the particle mass, , and the Morse constants via
:
Whereas the energy spacing between vibrational levels in the quantum harmonic oscillator
量子調和振動子 は、調和振動子, 古典調和振動子 の 量子力学, 量子力学 類似物です。任意の滑らかな ポテンシャル エネルギー, ポテンシャル は通常、安定した 平衡点 の近くで � ...
is constant at , the energy between adjacent levels decreases with increasing in the Morse oscillator. Mathematically, the spacing of Morse levels is
:
This trend matches the anharmonicity found in real molecules. However, this equation fails above some value of where is calculated to be zero or negative. Specifically,
: integer part.
This failure is due to the ''finite'' number of bound levels in the Morse potential, and some maximum that remains bound. For energies above , all the possible energy levels are allowed and the equation for is no longer valid.
Below , is a good approximation for the true vibrational structure in non-rotating diatomic molecules. In fact, the real molecular spectra are generally fit to the form1
:
in which the constants and can be directly related to the parameters for the Morse potential.
As is clear from dimensional analysis
In engineering and science, dimensional analysis is the analysis of the relationships between different physical quantities by identifying their base quantities (such as length, mass, time, and electric current) and units of measure (such as ...
, for historical reasons the last equation uses spectroscopic notation in which represents a wavenumber obeying , and not an angular frequency given by .
Morse/Long-range potential
An extension of the Morse potential that made the Morse form useful for modern (high-resolution) spectroscopy is the MLR ( Morse/Long-range) potential. The MLR potential is used as a standard for representing spectroscopic and/or virial data of diatomic molecules by a potential energy curve. It has been used on N2, Ca2, KLi, MgH, several electronic states of Li2, Cs2, Sr2, ArXe, LiCa, LiNa, Br2, Mg2, HF, HCl, HBr, HI, MgD, Be2, BeH, and NaH. More sophisticated versions are used for polyatomic molecules.See also
* Lennard-Jones potential * Molecular mechanicsReferences
*1 CRC Handbook of chemistry and physics, Ed David R. Lide, 87th ed, Section 9, ''SPECTROSCOPIC CONSTANTS OF DIATOMIC MOLECULES'' pp. 9–82 * * * * * * * * I.G. Kaplan, in Handbook of Molecular Physics and Quantum Chemistry, Wiley, 2003, p207. {{DEFAULTSORT:Morse Potential Chemical bonding Quantum chemistry Quantum models Quantum mechanical potentials