Migratory Insertion on:
[Wikipedia]
[Google]
[Amazon]
In
 CO insertion does not always involve migration. Treatment of CpFe(L)(CO)CH3 with 13CO yields a mix of both alkyl migration products and products formed by true insertion of bound carbonyls into the
CO insertion does not always involve migration. Treatment of CpFe(L)(CO)CH3 with 13CO yields a mix of both alkyl migration products and products formed by true insertion of bound carbonyls into the 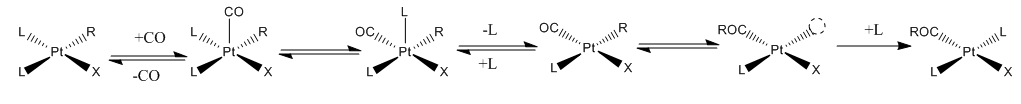


 The Cativa process catalytic cycle, shown above, includes both insertion and de-insertion steps. The oxidative addition reaction of
The Cativa process catalytic cycle, shown above, includes both insertion and de-insertion steps. The oxidative addition reaction of
Migratory Insertion
{{DEFAULTSORT:Migratory Insertion Organometallic chemistry Reaction mechanisms
organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:
:
Overview
In the migratory insertion, a ligand that is viewed as an anion (X) ligand in and a ligand that is viewed as neutral couple, generating a new anionic ligand. The anion and neutral ligands that react are adjacent. If the precursor complex is coordinatively saturated, migratory insertion often result in a coordinatively unsaturated product. A new (neutral) ligand can then react with themetal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
leading to a further insertion. The process can occur many times on a single metal, as in olefin polymerization
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
.
The anionic ligand can be: H− ( hydride), R− (alkyl), acyl, Ar− ( aryl), or OR− ( alkoxide). The ability of these groups to migrate is called their migratory aptitude Migratory aptitude is the relative ability of a migrating group to migrate in a rearrangement reaction. Migratory aptitudes vary in different reactions, depending on multiple factors.
In the Baeyer-Villiger reaction, the more substituted group, i ...
. The neutral ligand can be CO, alkene, alkyne, or in some cases, even carbene.
Diverse reactions apply to the migratory insertion. One mechanism involves the attack of the anionic ligand on the electrophilic part of the neutral ligand (the anionic ligand migrates to the neutral ligand). The other mechanism involves the neutral ligand inserts itself between the metal and the anionic ligand.
CO insertion
The insertion ofcarbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
into a metal-carbon bond to form an acyl group is the basis of carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbo ...
reactions, which provides many commercially useful products.
Mechanism
CO inserts into a metal- alkyl bond via migratory insertion. The key concept is that both the CO and the alkyl groups are ligands on the same metal. For example, the reaction of 13CO with Mn(CO)5CH3 exclusively form Mn(CO)4(13CO)COCH3. The alkyl group migrates intramolecularly to an adjacent CO ligand within the coordination sphere of the Mn(I) centre. Subsequent to the migration, the metal binds free CO (see figure below). : CO insertion does not always involve migration. Treatment of CpFe(L)(CO)CH3 with 13CO yields a mix of both alkyl migration products and products formed by true insertion of bound carbonyls into the
CO insertion does not always involve migration. Treatment of CpFe(L)(CO)CH3 with 13CO yields a mix of both alkyl migration products and products formed by true insertion of bound carbonyls into the methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs ...
group. Product distribution is influenced by the choice of solvent.
Alkyl derivatives of square planar complexes undergo CO insertions particularly readily. Insertion reactions on square planar complexes are of particular interest because of their industrial applications. Since square planar complexes are often coordinatively unsaturated, they are susceptible to formation of 5-coordinate adducts, which undergo migratory insertion readily. In most cases the in-plane migration pathway is preferred, but, unlike the nucleophilic pathway, it is inhibited by an excess of CO.

Effects on reaction rates
*Steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
strain – Increasing the steric strain of the chelate backbone in square planar complexes pushes the carbonyl and methyl groups closer together, increasing the reactivity of insertion reactions.
* Oxidation state – Oxidation of the metal tends to increase insertion reaction rates. The main rate-limiting step in the mechanism is the migration of the methyl group onto a carbonyl ligand, oxidizing the metal by imparting a greater partial positive charge on the acetyl carbon, and thus increasing the rate of reaction.
* Lewis acids – Lewis acids also increase the reaction rates, for reasons similar to metal oxidation increasing the positive charge on the carbon. Lewis acids bind to the CO oxygen and remove charge, increasing the electrophilicity of the carbon. This can increase the reaction rate by a factor of up to 108, and the complex formed is stable enough that the reaction proceeds even without additional CO to bind to the metal.
* Electronegativity of the leaving group - Increasing the electronegativity of the leaving alkyl group stabilizes the metal-carbon bond interaction and thus increases the activation energy required for migration, decreasing the reaction rate.
* ''Trans''-effect – Ligands in an octahedral or square planar complex are known to influence the reactivity of the group to which they are ''trans''. This ligand influence is often referred to as the ''trans''-influence, and it varies in intensity between ligands. A partial list of ''trans''-influencing ligands is as follows, from highest ''trans''-effect to lowest: aryl, alkyl > NR3 > PR3 > AsR3 > CO > Cl. Ligands with a greater ''trans''-influence impart greater electrophilicity to the active site. Increasing the electrophilicity of the CO group has been shown experimentally to greatly increase the reaction rate, while decreasing the electrophilicity of the methyl group slightly increases the reaction rate. This can be demonstrated by reacting a square planar PN)M(CO)(CH3)complex with CO, where PN is a bidentate phosphorus- or nitrogen-bound ligand. This reaction proceeds in much greater yield when the methyl group is ''trans''-P and the CO ''trans''-N, owing to the higher ''trans''-influence of the more electronegative nitrogen.
Reverse reaction
Decarbonylation of aldehydes, the reverse of CO insertion, is a well-recognized reaction: :RCHO → RH + CO The reaction is not widely practiced in part because the alkanes are less useful materials than are the aldehyde precursors. Furthermore, the reaction is not often conducted catalytically because the extruded CO can be slow to dissociate. Extrusion of CO from an organic aldehyde is most famously demonstrated using Wilkinson's catalyst: :RhCl(PPh3)3 + RCHO → RhCl(CO)(PPh3)2 + RH + PPh3 Please see Tsuji-Wilkinson Decarbonylation Reaction for an example of this elementary organometallic step in synthesisInsertion of other oxides
Many electrophilic oxides insert into metal carbon bonds; these includesulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
, carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, and nitric oxide. These reactions have limited or no practical significance, but are of historic interest. With transition metal alkyls, these oxides behave as electrophiles and insert into the bond between metals and their relatively nucleophilic alkyl ligands. As discussed in the article on Metal sulfur dioxide complexes, the insertion of SO2 has been examined in particular detail. SO2 inserts to give both ''O''-sulphinates and ''S''-sulphinates, depending on the metal centre. With square planar alkyl complexes, a pre-equilibrium is assumed involving formation of an adduct.
Insertion of alkenes into metal-carbon bonds
The insertion of alkenes into both metal-carbon is important. The insertion of ethylene and propylene into titanium alkyls is the cornerstone of Ziegler–Natta catalysis, the main source of polyethylene and polypropylene. The majority of this technology involves heterogeneous catalysts, but it is widely assumed that the principles and observations on homogeneous systems are applicable to the solid-state versions. Related technologies include the Shell Higher Olefin Process which produces detergent precursors. :
Mechanism
Factors affecting the rate of olefin insertions include the formation of the cyclic, planar, four-center transition state involving incipient formation of a bond between the metal and an olefin carbon. From this transition state, it can be seen that a partial positive charge forms on the β-carbon with a partial negative charge formed on the carbon initially bonded to the metal. This polarization explains the subsequently observed formation of the bond between the negatively charged carbon/hydrogen and the positively charged β-carbon as well as the simultaneous formation of the metal-α-carbon bond. This transition state also highlights the two factors that most strongly contribute to the rate of olefin insertion reactions: (i) orbital overlap of the alkyl group initially attached to the metal and (ii) the strength of the metal-alkyl bond. With greater orbital overlap between the partially positive β-carbon and the partially negative hydrogen/alkyl group carbon, the formation of the new C-C bond is facilitated. With increasing strength of the metal-alkyl bond, the breaking of the bond between the metal and the hydrogen/alkyl carbon bond to form the two new bonds with the α-carbon and β-carbon (respectively) is slower, thus decreasing the rate of the insertion reaction.Insertion of alkenes into M–H bonds
The insertion of alkenes into metal-hydrogen bonds is a key step inhydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
and hydroformylation reactions. The reaction involves the alkene and the hydride ligands combining within the coordination sphere of a catalyst. In hydrogenation, the resulting alkyl ligand combines with a second hydride to give the alkane. Analogous reactions apply to the hydrogenation of alkynes: an alkenyl ligand combines with a hydride to eliminate an alkene.

Mechanism
In terms of mechanism, the insertion of alkenes into M–H bond and into M–C bonds are described similarly. Both involve four-membered transition states that place the less substituted carbon on the metal. The reverse of olefin insertion into a metal-hydrogen bond isβ-hydride elimination
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo th ...
. The Principle of Microscopic Reversibility The principle of microscopic reversibility in physics and chemistry is twofold:
* First, it states that the microscopic detailed dynamics of particles and fields is time-reversible because the microscopic equations of motion are symmetric with respe ...
requires that the mechanism of β-hydride elimination follow the same pathway as the insertion of alkenes into metal hydride bonds. The first requirement for β-hydride elimination is the presence of a hydrogen at a position that is β with respect to the metal. β-elimination requires a vacant coordination position on the metal that will accommodate the hydrogen that is abstracted.
Industrial applications
Carbonylation
Two widely employed applications of migratory insertion of carbonyl groups are hydroformylation and the production ofacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
by carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbo ...
of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
. The former converts alkenes, hydrogen, and carbon monoxide into aldehydes. The production of acetic acid by carbonylation proceeds via two similar industrial processes. More traditional is the Monsanto acetic acid process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chemic ...
, which relies on a rhodium-iodine catalyst to transform methanol into acetic acid. This process has been superseded by the Cativa process which uses a related iridium catalyst, r(CO)2I2sup>− (1). By 2002, worldwide annual production of acetic acid stood at 6 million tons, of which approximately 60% is produced by the Cativa process.
: The Cativa process catalytic cycle, shown above, includes both insertion and de-insertion steps. The oxidative addition reaction of
The Cativa process catalytic cycle, shown above, includes both insertion and de-insertion steps. The oxidative addition reaction of methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h ...
with (1) involves the formal insertion of the iridium(I) centre into the carbon-iodine bond, whilst step (3) to (4) is an example of migratory insertion of carbon monoxide into the iridium-carbon bond. The active catalyst species is regenerated by the reductive elimination of acetyl iodide from (4), a de-insertion reaction.
Alkene polymerization
Industrial applications of alkene insertions include metal-catalyzed routes to polyethylene and polypropylene. Typically these conversions are catalyzed heterogeneously by titanium trichloride which are activated by aluminium alkyls. This technology is known as Ziegler–Natta catalysts. In these reactions, ethylene coordinates to titanium metal followed by its insertion. These steps can be repeated multiple times, potentially leading to high molecular weight polymers.References
External links
Migratory Insertion
{{DEFAULTSORT:Migratory Insertion Organometallic chemistry Reaction mechanisms