Membrane Scaling on:
[Wikipedia]
[Google]
[Amazon]
 Membrane scaling is when one or more sparingly soluble salts (e.g., calcium carbonate, calcium phosphate, etc.) precipitate and form a dense layer on the membrane surface in
Membrane scaling is when one or more sparingly soluble salts (e.g., calcium carbonate, calcium phosphate, etc.) precipitate and form a dense layer on the membrane surface in

 Membrane scaling is when one or more sparingly soluble salts (e.g., calcium carbonate, calcium phosphate, etc.) precipitate and form a dense layer on the membrane surface in
Membrane scaling is when one or more sparingly soluble salts (e.g., calcium carbonate, calcium phosphate, etc.) precipitate and form a dense layer on the membrane surface in reverse osmosis
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane, semi-permeable membrane to separate water molecules from other substances. RO applies pressure to overcome osmotic pressure that favors even distribu ...
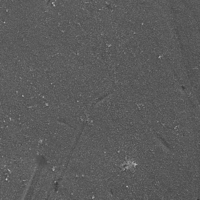
(RO) applications. Figures 1 and 2 show scanning electron microscopy
A scanning electron microscope (SEM) is a type of electron microscope that produces images of a sample by scanning the surface with a focused beam of electrons. The electrons interact with atoms in the sample, producing various signals that ...
(SEM) images of the RO membrane surface without and with scaling, respectively. Membrane scaling, like other types of membrane fouling
Membrane fouling is a process whereby a solution or a particle is deposited on a membrane surface or in membrane pores in a processes such as in a membrane bioreactor, reverse osmosis, forward osmosis, membrane distillation, ultrafiltration, micr ...
, increases energy costs due to higher operating pressure, and reduces permeate water production. Furthermore, scaling may damage and shorten the lifetime of membranes due to frequent membrane cleanings and therefore it is a major operational challenge in RO applications.
Membrane scaling can occur when sparingly soluble salts in RO concentrate become supersaturated
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a ...
, meaning their concentrations exceed their equilibrium (solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
) levels. In RO processes, the increased concentration of sparingly soluble salts in the concentrate is primarily caused by the withdrawal of permeate water from the feedwater. The ratio of permeate water to feedwater is known as recovery which is directly related to membrane scaling. Recovery needs to be as high as possible in RO installations to minimize specific energy consumption. However, at high recovery rates, the concentration of sparingly soluble salts in the concentrate can increase dramatically. For example, for 80% and 90% recovery, the concentration of salts in the concentrate can reach 5 and 10 times their concentration in the feedwater, respectively. If the calcium and phosphate concentrations in the RO feedwater are 200 mg/L and 5 mg/L, respectively, the concentrations in the RO concentrate will be 1000 mg/L and 50 mg/L at 90% recovery, exceeding the calcium phosphate solubility limit and resulting in calcium phosphate scaling.
It is important to note that membrane scaling is not only dependent on supersaturation but also on crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
kinetics, i.e., nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically def ...
and crystal growth
Crystal growth is a major stage of a crystallization, crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an ini ...
.
Scaling compounds encountered in RO
The most common salts that cause scaling in RO processes are: *Calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
* Calcium sulfate
Calcium sulfate (or calcium sulphate) is an inorganic salt with the chemical formula . It occurs in several hydrated forms; the anhydrous state (known as anhydrite) is a white crystalline solid often found in evaporite deposits. Its dihydrate ...
* Silica/ metal silicates
* Barium sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs in nature as the mineral barite, which is the main commercial source of ...
* Calcium phosphate
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are white ...
Scaling prediction methods
There are a number of indices available to determine the scaling tendency of sparingly soluble salts in a water solution. These indices provide information if a given scale-forming specie is undersaturated, saturated, or supersaturated. Scaling does not occur when a compound is undersaturated, while it will take place sooner or later when a compound is supersaturated. The most commonly used indices to predict scaling in RO applications are: * Saturation index (SI) where, IAP and Ksp are ion activity product andsolubility product
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reac ...
of the sparingly soluble salt, respectively. For instance, SI for calcium sulphate can be calculated as follows:
where, γ is activity coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same ( ...
. a2+and O42−are calcium and sulphate concentrations in mol/L, respectively.
* Supersaturation ratio (Sr)
where IAP and Ksp are ion activity product and solubility product of the sparingly soluble salt, respectively. For instance, Sr for calcium sulphate can be calculated as follows:
where, γ is activity coefficient. a2+and O42−are calcium and sulphate concentrations in mol/L, respectively.
* Langelier saturation index
Hard water is water that has a high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicar ...
(LSI)
LSI is used only for calcium carbonate scaling. On the other hand, SI and Sr are applicable for all compounds.
A positive value for each SI and LSI indicates that scaling may occur in RO, whereas a negative value implies that scaling will not occur. Similarly, scaling may occur when Sr>1, but not when Sr<1.
Scaling control in RO applications
There are several methods for preventing scaling in RO applications, including acidification of RO feed, lowering RO system recovery, and antiscalant addition. Acidification of RO feedwater was one of the first methods for tackling calcium carbonate scaling in RO processes. However, due to the risks associated with the use of acid, this method is becoming less common. Furthermore, acidification may not be effective for all types of scales; for example, it is very effective in preventing calcium carbonate scaling but not calcium sulphate scaling. Another method of preventing scaling is to operate RO at low recovery (ratio of permeate water to the feedwater). The recovery of the RO application is reduced in this approach to reduce the supersaturation level of the concentrate water to undersaturated conditions. Low recovery reduces the adverse effect of concentration polarization because there is less solute concentration on the membrane surface, reducing the potential for scale formation. This approach, however, is not very appealing or economical because it results in high specific energy consumption. Furthermore, the large amount of concentrate disposal is a problem. Antiscalants addition to the RO feed is one of the most widely applied strategies in term of scale control. Antiscalants can be used to increase the recovery of RO process and are primarily contains organic compounds such assulphonate
In organosulfur chemistry, a sulfonate is a salt (chemistry), salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the con ...
, phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing Functional group, groups, where R is an organic group (alkyl, aryl). If R is hydrogen then the compound is a Phosphite_ester#Chemistry_of_HP(O)(OR ...
, or carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s. The addition of antiscalants hinder the crystallization process, i.e., nucleation and/or growth phase of scaling compounds. Antiscalant prevent scale formation by three mechanisms, namely threshold inhibition, crystal modification and dispersion. Threshold inhibition is when antiscalant molecules adsorb on crystal nuclei and halt their nucleation process, whereas crystal modification and dispersion are the ability of antiscalants to stop the growth and/or agglomeration of crystals and particles. For silica scale, there is also an additional function where it prevents polymerisation of silica monomers, hence preventing the growth of silica polymers. There are various commercial antiscalants on the market such as Kurita, Avista, BASF etc. In RO applications, antiscalants are chosen based on the composition of the feedwater, and their doses are usually calculated using computer programs created by antiscalant manufacturers. For example, Avista has a chemical dosing software called AdvisorCI™, that is used to compute accurate dosing of chemicals in RO systems.
References
{{reflist Water treatment Fouling Membrane technology