|
Phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing Functional group, groups, where R is an organic group (alkyl, aryl). If R is hydrogen then the compound is a Phosphite_ester#Chemistry_of_HP(O)(OR)2, dialkyl phosphite, which is a different functional group. Phosphonic acids, typically handled as salts, are generally Volatility (chemistry), nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common Alcohol (chemistry), alcohols. Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide Roundup (herbicide), Roundup), and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic," in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2008. . In biochemistry and medicinal chemistry, phosphonate gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisphosphonates
Bisphosphonates are a class of drugs that prevent the loss of bone density, used to treat osteoporosis and similar diseases. They are the most commonly prescribed to treat osteoporosis. Evidence shows that they reduce the risk of fracture in post-menopausal women with osteoporosis. Bone tissue undergoes constant remodeling and is kept in balance (homeostasis) by osteoblasts creating bone and osteoclasts destroying bone. Bisphosphonates inhibit the digestion of bone by encouraging osteoclasts to undergo apoptosis, or cell death, thereby slowing bone loss. The uses of bisphosphonates include the prevention and treatment of osteoporosis, Paget's disease of bone, bone metastasis (with or without hypercalcemia), multiple myeloma, primary hyperparathyroidism, osteogenesis imperfecta, fibrous dysplasia, and other conditions that exhibit bone fragility. Chemical structure and mechanistic aspects The term bisphosphonate refers to the presence two phosphonate () groups. They are als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing Functional group, groups, where R is an organic group (alkyl, aryl). If R is hydrogen then the compound is a Phosphite_ester#Chemistry_of_HP(O)(OR)2, dialkyl phosphite, which is a different functional group. Phosphonic acids, typically handled as salts, are generally Volatility (chemistry), nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common Alcohol (chemistry), alcohols. Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide Roundup (herbicide), Roundup), and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic," in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2008. . In biochemistry and medicinal chemistry, phosphonate gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
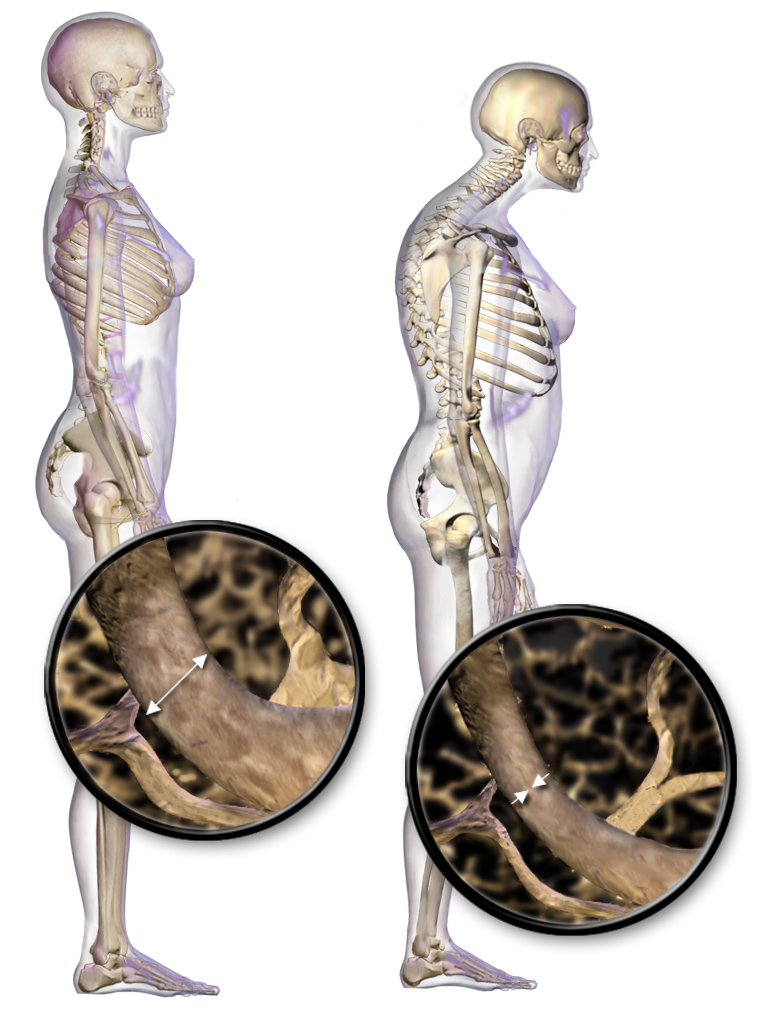
Osteoporosis
Osteoporosis is a systemic skeletal disorder characterized by low bone mass, micro-architectural deterioration of bone tissue leading to more porous bone, and consequent increase in Bone fracture, fracture risk. It is the most common reason for a broken bone among the Old age, elderly. Bones that commonly break include the vertebrae in the Vertebral column, spine, the bones of the forearm, the wrist, and the hip. Until a broken bone occurs there are typically no symptoms. Bones may weaken to such a degree that a break may occur with minor stress or spontaneously. After the broken bone heals, some people may have chronic pain and a decreased ability to carry out normal activities. Osteoporosis may be due to lower-than-normal peak bone mass, maximum bone mass and greater-than-normal bone loss. Bone loss increases after menopause in women due to lower levels of estrogen, and after andropause in older men due to lower levels of testosterone. Osteoporosis may also occur due to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hirao Coupling
In organic chemistry, Hirao coupling is a chemical reaction for the formation of Organophosphorus chemistry, carbon-phosphorus bonds using palladium Cross-coupling reaction, cross-coupling. Hirao coupling expands the scope of carbon-phosphorus bond formation from Alkyl group, alkyl (sp3) carbon-phosphorus bonds to sp2 (Alkene, alkenyl and Aryl group, aryl) carbon-phosphorus bonds. This builds on previous work by August Michaelis and Alexandr Arbuzov, who developed the Michaelis–Arbuzov reaction, Michaelis-Arbuzov reaction to deliver alkyl Phosphonate, phosphonates from Haloalkane, alkyl halides and Phosphinite, phosphinites in 1898. Earlier work used nickel halides (NiX2, X = Cl, Br) as catalysts at high temperatures to achieve vinyl phosphonates, but these reactions often proceeded in low yields and poor stereoselectivity. History The original work by Toshikazu Hirao et al. was published in 1980 and is the first example of synthesis of vinyl phosphonates using Palladium Cross- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX (nerve agent), VX nerve agents. Phosphorus, like nitrogen, is in pnictogen, group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt a v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminophosphonate
Aminophosphonates are organophosphorus compounds with the formula (RO)2P(O)CH2NR2. These compounds are structural analogues of amino acids in which a carboxylic moiety is replaced by phosphonic acid or related groups. Acting as antagonists of amino acids, they inhibit enzymes involved in amino acid metabolism and thus affect the physiological activity of the cell. These effects may be exerted as antibacterial, plant growth regulatory or neuromodulatory. They can act as ligands, and heavy metal complexes with aminophosphonates have medical applications. Phosphonates are more difficult to hydrolyse than phosphates. Some aminophosphonates degrade to aminomethylphosphonic acid. Preparation Aminophosphonates are often prepared by hydrophosphonylation, usually the condensation of imines and phosphorous acid. In the Pudovik reaction or Kabachnik–Fields reaction, the esters of phosphorous acid are employed, e.g. diphenylphosphite. Because these compounds are of pharmaceutical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tenofovir
Tenofovir disoproxil, sold under the brand name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together in combinations such as emtricitabine/tenofovir, efavirenz/emtricitabine/tenofovir, and elvitegravir/cobicistat/emtricitabine/tenofovir. It does not cure HIV/AIDS or hepatitis B. It is available by mouth as a tablet or powder. Common side effects include nausea, rash, diarrhea, headache, pain, depression, and weakness. Severe side effects include high blood lactate and an enlarged liver. There are no absolute contraindications. It is often recommended during pregnancy and appears to be safe. It is a nucleotide reverse transcriptase inhibitor and works by decreasing the abil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kabachnik–Fields Reaction
In organophosphorus chemistry, the Kabachnik–Fields reaction is a three-component organic reaction forming α-aminomethylphosphonates from an amine, a carbonyl compound, and a dialkyl phosphonate, (RO)2P(O)H (that are also called dialkylphosphites). Aminophosphonates are synthetic targets of some importance as phosphorus analogues of α-amino acids (a bioisostere). This multicomponent reaction was independently discovered by and Ellis K. Fields in 1952. The reaction is very similar to the two-component Pudovik reaction, which involves condensation of the phosphite and a preformed imine. : The first step in this reaction is the formation of an imine, followed by a hydrophosphonylation step where the phosphonate P–H bond across the C=N double bond. The starting carbonyl component is usually an aldehyde and sometimes a ketone. The reaction can be accelerated with a combination of dehydrating reagent and Lewis acid A Lewis acid (named for the American physical chemist Gi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorous Acid
Phosphorous acid (or phosphonic acid) is the Compound (chemistry), compound described by the chemical formula, formula . It is diprotic (readily ionizes two protons), not triprotic as might be suggested by its formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula , are called phosphonic acids. Nomenclature and tautomerism Solid has tetrahedral geometry about the central phosphorus atom, with a bond of 132 picometer, pm, one double bond of 148 pm and two longer single bonds of 154 pm. In common with other phosphorus oxides with bonds (e.g.hypophosphorous acid and dialkyl phosphites), it exists in equilibrium with an extremely minor tautomer . (In contrast, arsenous acid's major tautomer is the trihydroxy form.) IUPAC recommends that the trihydroxy form be called phosphorous acid, and the dihydroxy form phosphonic acid.. Only the reduced phosphorus c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clodronic Acid
Clodronic acid (INN) or clodronate disodium (Na2CH2Cl2O6P2) (USAN) is a first generation (non-nitrogenous) bisphosphonate. It is an anti-osteoporotic drug approved for the prevention and treatment of osteoporosis in post-menopausal women and men to reduce vertebral fractures, hyperparathyroidism, hypercalcemia in malignancy, multiple myeloma and fracture related pain because of its anti-inflammatory effects shown as a reduction in inflammatory markers like IL-1β, IL-6, and TNF-α. Medical uses A study comparing the analgesic effect of clodronic acid versus acetaminophen in osteoporotic vertebral fractures showed that clodronic acid provided more analgesia than 3 grams/day of acetaminophen. Clodronic acid is also used in experimental medicine to selectively deplete macrophages. Clodronic acid is approved for human use in Canada and Australia, the United Kingdom, where it is marketed as Bonefos, Loron, Clodron and in Italy as Clasteon, Difosfonal, Osteostab and several generi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphite Ester
file:Phosphite.svg, The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an Alcohol (chemistry), alcohol. For alkyl alcohols the displaced chloride ion can attack the phosphite, causing dealkylation to give a dialkylphosphite and an Organochlorine chemistry, organochlorine compound. The overall reaction is as follows: :PCl3 + 3 C2H5OH → (C2H5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyphosate
Glyphosate (IUPAC name: ''N''-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide and crop desiccant. It is an organophosphorus compound, specifically a phosphonate, which acts by EPSP inhibitor, inhibiting the plant enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSP). Glyphosate-based herbicides (GBHs) are used to kill weeds, especially annual Forbs, broadleaf weeds and grasses that compete with crops. Monsanto brought it to market for agricultural use in 1974 under the trade name Roundup (herbicide), ''Roundup''. Monsanto's last commercially relevant United States patent expired in 2000. Farmers quickly adopted glyphosate for agricultural weed control, especially after Monsanto introduced glyphosate-resistant Roundup Ready crops, enabling farmers to kill weeds without killing their crops. In 2007, glyphosate was the most used herbicide in the United States' agricultural sector and the second-most used (after 2,4-Dichlorophenoxyacetic acid, 2,4-D) in home ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |