Mass Spectrum Analysis on:
[Wikipedia]
[Google]
[Amazon]
Mass spectral interpretation is the method employed to identify the chemical formula, characteristic fragment patterns and possible fragment ions from the mass spectra. Mass spectra is a plot of relative abundance against mass-to-charge ratio. It is commonly used for the identification of organic compounds from




 Branched alkanes have somewhat weaker molecular ion peaks in the spectra. They tend to fragment at the branched point. For the 2,3-dimethylbutane, an isopropyl cation peak (m/z=43) is very strong.
Branched alkanes have somewhat weaker molecular ion peaks in the spectra. They tend to fragment at the branched point. For the 2,3-dimethylbutane, an isopropyl cation peak (m/z=43) is very strong.
 Cycloalkanes have relatively intense molecular ion peaks (two bonds have to break). Alkene fragmentation peaks are often most significant mode. Loss of “CH2CH2“ (= 28) is common, if present. However, for the substituted cycloalkanes, they prefer to form the cycloalkyl cations by cleavage at the branched points.
Cycloalkanes have relatively intense molecular ion peaks (two bonds have to break). Alkene fragmentation peaks are often most significant mode. Loss of “CH2CH2“ (= 28) is common, if present. However, for the substituted cycloalkanes, they prefer to form the cycloalkyl cations by cleavage at the branched points.

 McLafferty-like rearrangements are possible (similar to carbonyl pi bonds). Again, bond migration is possible.
McLafferty-like rearrangements are possible (similar to carbonyl pi bonds). Again, bond migration is possible.
 Cyclohexenes often undergo retro Diels-Alder reactions.
Cyclohexenes often undergo retro Diels-Alder reactions.
 Alkyl substituted benzenes can fragment via the kinetic controlled process to form C6H5+, C6H6+ ions.
Another common mode of fragmentation is the McLafferty rearrangement, which requires the alkyl chain length to be at least longer than 3 carbons.
Alkyl substituted benzenes can fragment via the kinetic controlled process to form C6H5+, C6H6+ ions.
Another common mode of fragmentation is the McLafferty rearrangement, which requires the alkyl chain length to be at least longer than 3 carbons.

 Another common fragmentation mode is dehydration (M-18). For longer chain alcohols, a McLafferty type rearrangement can produce water and ethylene (M -46).
Another common fragmentation mode is dehydration (M-18). For longer chain alcohols, a McLafferty type rearrangement can produce water and ethylene (M -46).
 Cyclic alcohols tend to show stronger M+ peaks than linear chains. And they follow similar fragmentation pathways: Alpha cleavage and dehydration.
Cyclic alcohols tend to show stronger M+ peaks than linear chains. And they follow similar fragmentation pathways: Alpha cleavage and dehydration.

 Aromatic ethers can generate the C6H5O+ ion by loss of the alkyl group rather than H; this can expel CO as in the phenolic degradation.
Aromatic ethers can generate the C6H5O+ ion by loss of the alkyl group rather than H; this can expel CO as in the phenolic degradation.

 β-cleavage is a characteristic mode of carbonyl compounds' fragmentation due to the resonance stabilization.
β-cleavage is a characteristic mode of carbonyl compounds' fragmentation due to the resonance stabilization.
 For longer chain carbonyl compounds (carbon number is bigger than 4), McLafferty rearrangements are dominant.
For longer chain carbonyl compounds (carbon number is bigger than 4), McLafferty rearrangements are dominant.
 According to these fragmentation patterns, the characteristic peaks of carbonyl compounds are summarized in the following table.
For aromatic carbonyl compounds, Alpha-cleavages are favorable primarily to lose G· (M – 1,15, 29…) to form the C6H5CO+ ion (m/z=105), which can further lose CO (m/z= 77) and HCCH (m/z=51).
According to these fragmentation patterns, the characteristic peaks of carbonyl compounds are summarized in the following table.
For aromatic carbonyl compounds, Alpha-cleavages are favorable primarily to lose G· (M – 1,15, 29…) to form the C6H5CO+ ion (m/z=105), which can further lose CO (m/z= 77) and HCCH (m/z=51).

 Aromatic amines have intense molecular ion peaks. For anilines, they prefer to lose a hydrogen atom before the expulsion of HCN.
Aromatic amines have intense molecular ion peaks. For anilines, they prefer to lose a hydrogen atom before the expulsion of HCN.



electron ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of th ...
mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
. Organic chemists obtain mass spectra of chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s as part of structure elucidation and the analysis is part of many organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
curricula.
Mass spectra generation
Electron ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of th ...
(EI) is a type of mass spectrometer ion source
An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Electron ionization
Elect ...
in which a beam of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s interacts with a gas phase molecule M to form an ion according to
:
with a molecular ion . The superscript "+" indicates the ion charge and the superscript "•" indicates an unpaired electron
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contai ...
of the radical ion
In organic chemistry, a radical anion is a Radical (chemistry), free radical species that carries a charge (chemistry), negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e ...
. The energy of the electron beam is typically 70 electronvolt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an Voltage, electric potential difference of one volt in vacuum ...
s and the ionization process typically produces extensive fragmentation of the chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s of the molecule.
Due to the high vacuum pressure in the ionization chamber, the mean free path of molecules are varying from 10 cm to 1 km and then the fragmentations are unimolecular processes. Once the fragmentation is initiated, the electron is first excited from the site with the lowest ionization energy. Since the order of the electron energy is non-bonding electrons > pi bond electrons > sigma bond electrons, the order of ionization preference is non-bonding electrons > pi bond electrons > sigma bond electrons.
The peak in the mass spectrum with the greatest intensity is called the base peak. The peak corresponding to the molecular ion is often, but not always, the base peak. Identification of the molecular ion can be difficult. Examining organic compounds, the relative intensity of the molecular ion peak diminishes with branching and with increasing mass in a homologous series. In the spectrum for toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
for example, the molecular ion peak is located at 92 m/z corresponding to its molecular mass
The molecular mass () is the mass of a given molecule, often expressed in units of daltons (Da). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The derived quan ...
. Molecular ion peaks are also often preceded by an M-1 or M-2 peak resulting from loss of a hydrogen radical or dihydrogen, respectively. Here, M refers to the molecular mass of the compound. In the spectrum for toluene, a hydrogen radical (proton-electron pair) is lost, forming the M-1 (91) peak.
Peaks with mass less than the molecular ion are the result of fragmentation of the molecule. Many reaction pathways exist for fragmentation, but only newly formed cations will show up in the mass spectrum, not radical fragments or neutral fragments. Metastable peaks are broad peaks with low intensity at non-integer mass values. These peaks result from ions with lifetimes shorter than the time needed to traverse the distance between ionization chamber and the detector.
Molecular formula determination
Nitrogen rule
Thenitrogen rule The nitrogen rule states that organic compounds containing exclusively hydrogen, carbon, nitrogen, oxygen, silicon, phosphorus, sulfur, and the halogens either have (1) an ''odd nominal mass'' that indicates an ''odd number'' of nitrogen atoms are p ...
states that organic molecules that contain hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
, or the halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s have an odd nominal mass if they have an odd number of nitrogen atoms or an even mass if they have an even number of nitrogen atoms are present. The nitrogen rule is true for structures in which all of the atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s in the molecule have a number of covalent bonds equal to their standard valency, counting each sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
and pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
as a separate covalent bond.
Rings rule
From degree of unsaturation principles, molecules containing only carbon, hydrogen, halogens, nitrogen, and oxygen follow the formula : where C is the number of carbons, H is the number of hydrogens, X is the number of halogens, and N is the number of nitrogen.Even electron rule
The even electron rule states that ions with an even number of electrons (cations but not radical ions) tend to form even-electron fragment ions and odd-electron ions (radical ions) form odd-electron ions or even-electron ions. Even-electron species tend to fragment to another even-electron cation and a neutral molecule rather than two odd-electron species.Stevenson's rules
The more stable the product cation, the more abundant the corresponding decomposition process. Several theories can be utilized to predict the fragmentation process, such as the electron octet rule, the resonance stabilization and hyperconjugation and so on.Rule of 13
The Rule of 13 is a simple procedure for tabulating possiblechemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
for a given molecular mass. The first step in applying the rule is to assume that only carbon and hydrogen are present in the molecule and that the molecule comprises some number of CH "units" each of which has a nominal mass of 13. If the molecular weight of the molecule in question is ''M'', the number of possible CH units is ''n'' and
:
where r is the remainder. The base formula for the molecule is
:
and the degree of unsaturation is
:
A negative value of ''u'' indicates the presence of heteroatoms in the molecule and a half-integer value of ''u'' indicates the presence of an odd number of nitrogen atoms. On addition of heteroatoms, the molecular formula is adjusted by the equivalent mass of carbon and hydrogen. For example, adding N requires removing CH2 and adding O requires removing CH4.
Isotope effects
Isotope peaks within a spectrum can help in structure elucidation. Compounds containing halogens (especiallychlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
and bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
) can produce very distinct isotope peaks. The mass spectrum of methylbromide has two prominent peaks of equal intensity at ''m/z'' 94 (M) and 96 (M+2) and then two more at 79 and 81 belonging to the bromine fragment.
Even when compounds only contain elements with less intense isotope peaks (carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
or oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
), the distribution of these peaks can be used to assign the spectrum to the correct compound. For example, two compounds with identical mass of 150 Da, C8H12N3+ and C9H10O2+, will have two different M+2 intensities which makes it possible to distinguish between them.
Fragmentation
The fragmentation pattern of the spectra beside the determination of the molar weight of an unknown compound also suitable to give structural information, especially in combination with the calculation of the degree of unsaturation from themolecular formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as paren ...
(when available). Neutral fragments frequently lost are carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
, ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
, water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
, ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, and hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
. There are several fragmentation processes, as follows.
α - cleavage
Fragmentation arises from a homolysis processes. This cleavage results from the tendency of the unpaired electron from the radical site to pair up with an electron from another bond to an atom adjacent to the charge site, as illustrated below. This reaction is defined as a homolytic cleavage since only a single electron is transferred. The driving forces for such reaction is the electron donating abilities of the radical sites: N > S, O,π > Cl, Br > H. An example is the cleavage of carbon-carbon bonds next to aheteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
. In this depiction, single-electron movements are indicated by a single-headed arrow.
Sigma bond cleavage
The ionization of alkanes weakens the C-C bond, ultimately resulting in the decomposition. As the bond breaks, a charged, even electron species (R+) and a neutral radical species (R•) are generated. Highly substituted carbocations are more stable than the nonsubstituted ones. An example is depicted below.
Inductive cleavage
This reaction results from the inductive effect of the radical sites, as depicted below. This reaction is defined as a heterolytic cleavage since a pair of electrons is transferred. The driving forces for such reaction are the electronegativities of the radical sites: halogens > O, S >> N, C. this reaction is less favored than radical-site reactions.
McLafferty rearrangement
The McLafferty rearrangement can occur in a molecule containing a keto-group and involves β-cleavage, with the gain of the γ-hydrogen atom. Ion-neutral complex formation involves bond homolysis or bond heterolysis, in which the fragments do not have enough kinetic energy to separate and, instead, reaction with one another like an ion-molecule reaction.Hydrogen rearrangement to a saturated heteroatom
The “1,5 ” hydrogen shift cause transfer of one γ- hydrogen to a radical site on a saturated heteroatom. The same requirements for McLafferty rearrangement apply to hydrogen rearrangement to a saturated heteroatom. Such rearrangement initiates charge-site reaction, resulting in the formation of an odd electron ion and a small neutral molecule ( water, or acid and so on). For alcohols, this heterolytic cleavage releases a water molecule. Since the charge-site reactions are dominant in the less bulky alcohols, this reaction is favored for alcohols as primary > secondary > tertiary.Double-hydrogen rearrangement
The “1,5 ” hydrogen shift cause transfer of two γ- hydrogen to two radical sites on two different unsaturated atoms. The same requirements for McLafferty rearrangement apply to double-hydrogen rearrangement. This reaction is observed for three unsaturated functional groups, namely thioesters, esters and amides.Ortho rearrangement
The “1,5 ” hydrogen shift cause transfer of two γ- hydrogen to two radical sites on two different unsaturated atoms. The same requirements for The “1,5 ” hydrogen shift occur between proper substituents in the ortho positions of the aromatic rings. The same requirements for McLafferty rearrangement apply to ortho rearrangement except for the strong α,β carbon-carbon double bond. Such rearrangement initiates charge-site reaction, resulting in the formation of an odd electron ion and a small neutral molecule ( water, or HCl and so on). This reaction can be utilized to differentiate ortho from para and meta isomersMcLafferty rearrangement apply to double-hydrogen rearrangement. This reaction is observed for three unsaturated functional groups, namely thioesters, esters and amides.
Retro-Diels-Alder reaction
This reaction occurs mainly in cyclohexene and its derivatives. Upon ionization, the pi electrons are excited and generate a charge site and a radical site. Following this, two successive α cleavages yield a butadiene radical and a neutral ethene since ethene has a higher ionisation energy than butadiene ( Stevenson's rules).Cycloelimination reaction
This reaction occurs mainly in four-membered cyclic molecules. Once ionized, it produces a distonic ion and then further fragments to yield an ethene radical ion and a neutral ethene molecule.Fragmentation patterns of specific compound classes
Alkanes
For linear alkanes, molecular ion peaks are often observed. However, for long chain compounds, the intensity of the molecular ion peaks are often weak. Linear fragments often differ by 14 Da (CH2 = 14). For example, hexane fragmentation patterns. The m/z=57 butyl cation is the base peak, and other most abundant peaks in the spectrum are alkyl carbocations at m/z=15, 29, 43 Da. Branched alkanes have somewhat weaker molecular ion peaks in the spectra. They tend to fragment at the branched point. For the 2,3-dimethylbutane, an isopropyl cation peak (m/z=43) is very strong.
Branched alkanes have somewhat weaker molecular ion peaks in the spectra. They tend to fragment at the branched point. For the 2,3-dimethylbutane, an isopropyl cation peak (m/z=43) is very strong.
 Cycloalkanes have relatively intense molecular ion peaks (two bonds have to break). Alkene fragmentation peaks are often most significant mode. Loss of “CH2CH2“ (= 28) is common, if present. However, for the substituted cycloalkanes, they prefer to form the cycloalkyl cations by cleavage at the branched points.
Cycloalkanes have relatively intense molecular ion peaks (two bonds have to break). Alkene fragmentation peaks are often most significant mode. Loss of “CH2CH2“ (= 28) is common, if present. However, for the substituted cycloalkanes, they prefer to form the cycloalkyl cations by cleavage at the branched points.

Alkenes
Alkenes often produce stronger molecular ion peaks than alkanes due to the lower ionization energy of a pi electron than a σ electron. After the ionization, double bonds can migrate easily, resulting in almost impossible determination of isomers. Allylic cleavage is most significant fragmentation mode due to resonance stabilization. McLafferty-like rearrangements are possible (similar to carbonyl pi bonds). Again, bond migration is possible.
McLafferty-like rearrangements are possible (similar to carbonyl pi bonds). Again, bond migration is possible.
 Cyclohexenes often undergo retro Diels-Alder reactions.
Cyclohexenes often undergo retro Diels-Alder reactions.
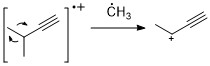
Alkynes
Similar to alkenes, alkynes often show strong molecular ion peak. Propargylic cleavage is a most significant fragmentation mode.
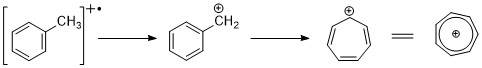
Aromatic hydrocarbons
Aromatic hydrocarbons show distinct molecular ion peak.benzylic cleavage is pretty common. When alkyl groups are attached to the ring, a favorable mode of cleavage is to lose a H-radical to form the tropylium cation (m/z 91). Alkyl substituted benzenes can fragment via the kinetic controlled process to form C6H5+, C6H6+ ions.
Another common mode of fragmentation is the McLafferty rearrangement, which requires the alkyl chain length to be at least longer than 3 carbons.
Alkyl substituted benzenes can fragment via the kinetic controlled process to form C6H5+, C6H6+ ions.
Another common mode of fragmentation is the McLafferty rearrangement, which requires the alkyl chain length to be at least longer than 3 carbons.

Alcohols
Alcohols generally have weak molecular ion peaks due to the strong electronegativity of oxygen. “Alpha” cleavage is common due to the resonance stabilization. The largest alkyl group will be lost. Another common fragmentation mode is dehydration (M-18). For longer chain alcohols, a McLafferty type rearrangement can produce water and ethylene (M -46).
Another common fragmentation mode is dehydration (M-18). For longer chain alcohols, a McLafferty type rearrangement can produce water and ethylene (M -46).
 Cyclic alcohols tend to show stronger M+ peaks than linear chains. And they follow similar fragmentation pathways: Alpha cleavage and dehydration.
Cyclic alcohols tend to show stronger M+ peaks than linear chains. And they follow similar fragmentation pathways: Alpha cleavage and dehydration.
Phenol
Phenol exhibit a strong molecular ion peak. Loss of H· is observed (M – 1), CO (M – 28) and formyl radical (HCO·, M – 29) is common observed.
Ether
Ethers produce slightly more intense molecular ion peaks compared to the corresponding alcohols or alkanes. There are two common cleavage modes. α-cleavage and C-O bond cleavage. Aromatic ethers can generate the C6H5O+ ion by loss of the alkyl group rather than H; this can expel CO as in the phenolic degradation.
Aromatic ethers can generate the C6H5O+ ion by loss of the alkyl group rather than H; this can expel CO as in the phenolic degradation.

Carbonyl compounds
There are five types of carbonyl compounds, including aldehydes, ketones, carboxylic acids and esters. The principal fragmentation modes are described as follows: Alpha-cleavage can occur on either side of the carbonyl functional group since an oxygen lone pair can stabilize the positive charge. β-cleavage is a characteristic mode of carbonyl compounds' fragmentation due to the resonance stabilization.
β-cleavage is a characteristic mode of carbonyl compounds' fragmentation due to the resonance stabilization.
 For longer chain carbonyl compounds (carbon number is bigger than 4), McLafferty rearrangements are dominant.
For longer chain carbonyl compounds (carbon number is bigger than 4), McLafferty rearrangements are dominant.
 According to these fragmentation patterns, the characteristic peaks of carbonyl compounds are summarized in the following table.
For aromatic carbonyl compounds, Alpha-cleavages are favorable primarily to lose G· (M – 1,15, 29…) to form the C6H5CO+ ion (m/z=105), which can further lose CO (m/z= 77) and HCCH (m/z=51).
According to these fragmentation patterns, the characteristic peaks of carbonyl compounds are summarized in the following table.
For aromatic carbonyl compounds, Alpha-cleavages are favorable primarily to lose G· (M – 1,15, 29…) to form the C6H5CO+ ion (m/z=105), which can further lose CO (m/z= 77) and HCCH (m/z=51).

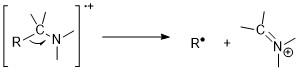
Amines
Amines follow nitrogen rule. Odd molecular ion mass-to-charge ratio suggests existence of odd numbers of nitrogens. Nonetheless, molecular ion peaks are weak in aliphatic amines due to the ease of fragmentation next to amines. Alpha-cleavage reactions are the most important fragmentation mode for amines; for 1° n-aliphatic amines, there is an intense peak at m/z 30. Aromatic amines have intense molecular ion peaks. For anilines, they prefer to lose a hydrogen atom before the expulsion of HCN.
Aromatic amines have intense molecular ion peaks. For anilines, they prefer to lose a hydrogen atom before the expulsion of HCN.

Nitriles
The principle fragmentation mode is the loss of an H-atom (M – 1) from the carbon next to the CN group due to the resonance stabilization. McLafferty rearrangement can be observed when they have longer chain lengths.
Nitro compounds
The aliphatic nitro compounds normally show weak molecular ion peaks, while the aromatic nitro compounds give a strong peak. Common degradation mode is loss of NO+ and NO2+.
Electrospray and atmospheric pressure chemical ionization
Electrospray andatmospheric pressure chemical ionization
Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry which utilizes gas-phase ion-molecule reactions at atmospheric pressure (105 Pa), commonly coupled with high-performance liquid chromatography (HPL ...
have different rules for spectrum interpretation due to the different ionization mechanisms.
See also
* Component Detection Algorithm (CODA), an algorithm used in mass spectrometry data analysis * List of mass spectrometry softwareReferences
{{DEFAULTSORT:Mass-Spectrum Analysis Mass spectrometry