Macrophages (; abbreviated M
Žå, M╬” or MP) are a type of
white blood cell
White blood cells (scientific name leukocytes), also called immune cells or immunocytes, are cells of the immune system that are involved in protecting the body against both infectious disease and foreign entities. White blood cells are genera ...
of the
innate immune system
The innate immune system or nonspecific immune system is one of the two main immunity strategies in vertebrates (the other being the adaptive immune system). The innate immune system is an alternate defense strategy and is the dominant immune s ...
that engulf and digest pathogens, such as
cancer cell
Cancer cells are cells that divide continually, forming solid tumors or flooding the blood or lymph with abnormal cells. Cell division is a normal process used by the body for growth and repair. A parent cell divides to form two daughter cells, an ...
s,
microbe
A microorganism, or microbe, is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from antiquity, with an early attestation in ...
s, cellular debris and foreign substances, which do not have proteins that are specific to healthy body cells on their surface.
This self-protection method can be contrasted with that employed by
Natural Killer cells
Natural killer cells, also known as NK cells, are a type of cytotoxic lymphocyte critical to the innate immune system. They are a kind of large granular lymphocytes (LGL), and belong to the rapidly expanding family of known innate lymphoid cells ...
. This process of engulfment and digestion is called
phagocytosis
Phagocytosis () is the process by which a cell (biology), cell uses its plasma membrane to engulf a large particle (Ōēź 0.5 ╬╝m), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs ph ...
; it acts to defend the host against infection and injury.
Macrophages are found in essentially all tissues,
where they patrol for potential
pathogen
In biology, a pathogen (, "suffering", "passion" and , "producer of"), in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a Germ theory of d ...
s by
amoeboid movement
Amoeboid movement is the most typical mode of locomotion in adherent eukaryotic cells. It is a crawling-like type of movement accomplished by protrusion of cytoplasm of the cell involving the formation of pseudopodia ("false-feet") and posterio ...
. They take various forms (with various names) throughout the body (e.g.,
histiocyte
A histiocyte is a vertebrate cell that is part of the mononuclear phagocyte system (also known as the reticuloendothelial system or lymphoreticular system). The mononuclear phagocytic system is part of the organism's immune system. The histiocy ...
s,
Kupffer cell
Kupffer cells, also known as stellate macrophages and KupfferŌĆōBrowicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. K ...
s,
alveolar macrophage
An alveolar macrophage, pulmonary macrophage, (or dust cell, or dust eater) is a type of macrophage, a phagocytosis#Professional phagocytic cells, professional phagocyte, found in the airways and at the level of the pulmonary alveolus, alveoli in ...
s,
microglia
Microglia are a type of glia, glial cell located throughout the brain and spinal cord of the central nervous system (CNS). Microglia account for about around 5ŌĆō10% of cells found within the brain. As the resident macrophage cells, they act as t ...
, and others), but all are part of the
mononuclear phagocyte system
In immunology, the mononuclear phagocyte system or mononuclear phagocytic system (MPS), also known as the macrophage system, is a part of the immune system that consists of the Phagocyte, phagocytic cells located in reticular connective tissue. T ...
. Besides phagocytosis, they play a critical role in nonspecific defense (
innate immunity
The innate immune system or nonspecific immune system is one of the two main immunity strategies in vertebrates (the other being the adaptive immune system). The innate immune system is an alternate defense strategy and is the dominant immune s ...
) and also help initiate specific defense mechanisms (
adaptive immunity
The adaptive immune system (AIS), also known as the acquired immune system, or specific immune system is a subsystem of the immune system that is composed of specialized cells, organs, and processes that eliminate pathogens specifically. The ac ...
) by recruiting other immune cells such as
lymphocyte
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include T cells (for cell-mediated and cytotoxic adaptive immunity), B cells (for humoral, antibody-driven adaptive immunity), an ...
s. For example, they are important as
antigen presenters to
T cell
T cells (also known as T lymphocytes) are an important part of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell ...
s. In humans, dysfunctional macrophages cause severe diseases such as
chronic granulomatous disease
Chronic granulomatous disease (CGD), also known as BridgesŌĆōGood syndrome, chronic granulomatous disorder, and Quie syndrome, is a diverse group of hereditary diseases in which certain cells of the immune system have difficulty forming the react ...
that result in frequent infections.
Beyond increasing
inflammation
Inflammation (from ) is part of the biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. The five cardinal signs are heat, pain, redness, swelling, and loss of function (Latin ''calor'', '' ...
and stimulating the immune system, macrophages also play an important
anti-inflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation, fever or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs reduce pain by inhibiting mechan ...
role and can decrease immune reactions through the release of
cytokines
Cytokines () are a broad and loose category of small proteins (~5ŌĆō25 kDa) important in cell signaling.
Cytokines are produced by a broad range of cells, including immune cells like macrophages, B cell, B lymphocytes, T cell, T lymphocytes ...
. Macrophages that encourage inflammation are called M1 macrophages, whereas those that decrease inflammation and encourage tissue repair are called M2 macrophages. This difference is reflected in their metabolism; M1 macrophages have the unique ability to metabolize
arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidinium, guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (ŌłÆCO2ŌłÆ) a ...
to the "killer" molecule
nitric oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes den ...
, whereas M2 macrophages have the unique ability to metabolize arginine to the "repair" molecule
ornithine
Ornithine is a non-proteinogenic ╬▒-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of th ...
. However, this dichotomy has been recently questioned as further complexity has been discovered. Macrophages are widely thought of as highly plastic and fluid cells, with a fluctuating phenotype.
Human macrophages are about in diameter and are produced by the differentiation of
monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
s in tissues. They can be identified using
flow cytometry
Flow cytometry (FC) is a technique used to detect and measure the physical and chemical characteristics of a population of cells or particles.
In this process, a sample containing cells or particles is suspended in a fluid and injected into the ...
or
immunohistochemical staining
Immunohistochemistry is a form of immunostaining. It involves the process of selectively identifying antigens in cells and tissue, by exploiting the principle of antibodies binding specifically to antigens in biological tissues. Albert Hewett ...
by their specific expression of proteins such as
CD14
CD14 ( cluster of differentiation 14) is a human protein made mostly by macrophages as part of the innate immune system. It helps to detect bacteria in the body by binding lipopolysaccharide (LPS), a pathogen-associated molecular pattern (PAMP). ...
,
CD40
Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on T helper cell, TH cells to CD40 activates antigen presenting cells and i ...
,
CD11b
Integrin alpha M (ITGAM) is one protein subunit that forms heterodimeric integrin alpha-M beta-2 (╬▒M╬▓2) molecule, also known as '' macrophage-1 antigen'' (Mac-1) or '' complement receptor 3'' (CR3). ITGAM is also known as CR3A, and cluster of di ...
,
CD64,
F4/80
EGF-like module-containing mucin-like hormone receptor-like 1 also known as F4/80 is a protein encoded by the ''ADGRE1'' gene.
EMR1 is a member of the adhesion GPCR family characterized by an extended extracellular region containing EGF-like ...
(mice)/
EMR1 (human),
lysozyme
Lysozyme (, muramidase, ''N''-acetylmuramide glycanhydrolase; systematic name peptidoglycan ''N''-acetylmuramoylhydrolase) is an antimicrobial enzyme produced by animals that forms part of the innate immune system. It is a glycoside hydrolase ...
M,
MAC-1/MAC-3 and
CD68
CD68 ( Cluster of Differentiation 68) is a protein highly expressed by cells in the monocyte lineage (e.g., monocytic phagocytes, osteoclasts), by circulating macrophages, and by tissue macrophages (e.g., Kupffer cells, microglia).
Structure and ...
.
Macrophages were first discovered and named by
├ēlie Metchnikoff
Ilya Ilyich Mechnikov (; ŌĆō 15 July 1916), also spelled ├ēlie Metchnikoff, was a zoologist from the Russian Empire of Moldavian noble ancestry and alshereat archive.org best known for his research in immunology (study of immune systems) and ...
, a Russian Empire zoologist, in 1884.
Structure
Types
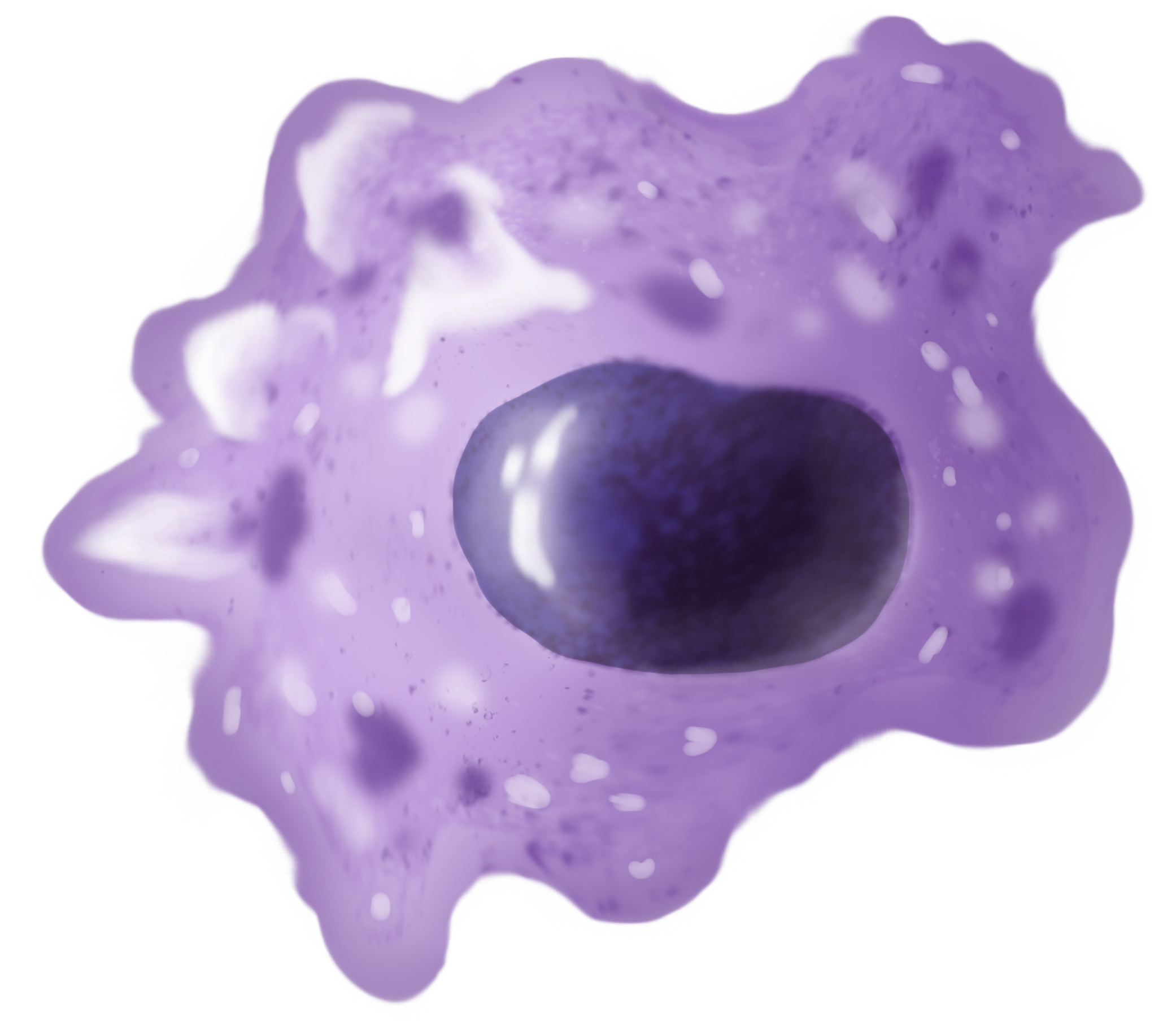
A majority of macrophages are stationed at strategic points where microbial invasion or accumulation of foreign particles is likely to occur. These cells together as a group are known as the
mononuclear phagocyte system
In immunology, the mononuclear phagocyte system or mononuclear phagocytic system (MPS), also known as the macrophage system, is a part of the immune system that consists of the Phagocyte, phagocytic cells located in reticular connective tissue. T ...
and were previously known as the reticuloendothelial system. Each type of macrophage, determined by its location, has a specific name:
Investigations concerning Kupffer cells are hampered because in humans, Kupffer cells are only accessible for immunohistochemical analysis from biopsies or autopsies. From rats and mice, they are difficult to isolate, and after purification, only approximately 5 million cells can be obtained from one mouse.
Macrophages can express
paracrine
In cellular biology, paracrine signaling is a form of cell signaling, a type of cellular communication (biology), cellular communication in which a Cell (biology), cell produces a signal to induce changes in nearby cells, altering the behaviour of ...
functions within organs that are specific to the function of that organ. In the
testis
A testicle or testis ( testes) is the gonad in all male bilaterians, including humans, and is Homology (biology), homologous to the ovary in females. Its primary functions are the production of sperm and the secretion of Androgen, androgens, p ...
, for example, macrophages have been shown to be able to interact with
Leydig cells
Leydig cells, also known as interstitial cells of the testes and interstitial cells of Leydig, are found adjacent to the seminiferous tubules in the testicle and produce testosterone in the presence of luteinizing hormone (LH). They are polyhedral ...
by secreting
25-hydroxycholesterol 25-Hydroxycholesterol is a derivative of cholesterol, which plays a role in various biological processes in humans and other species. It is involved in cholesterol metabolism, antivirus process, inflammatory and immune response, and survival signali ...
, an
oxysterol
An oxysterol is a derivative of cholesterol obtained by Redox, oxidation involving enzymes and / or pro-oxidants. Such compounds play important roles in various biological processes such as cholesterol homeostasis, lipid metabolism (sphingolipids, ...
that can be converted to
testosterone
Testosterone is the primary male sex hormone and androgen in Male, males. In humans, testosterone plays a key role in the development of Male reproductive system, male reproductive tissues such as testicles and prostate, as well as promoting se ...
by neighbouring Leydig cells.
Also, testicular macrophages may participate in creating an immune privileged environment in the testis, and in mediating infertility during inflammation of the testis.
Cardiac resident macrophages participate in electrical conduction via
gap junction
Gap junctions are membrane channels between adjacent cells that allow the direct exchange of cytoplasmic substances, such small molecules, substrates, and metabolites.
Gap junctions were first described as ''close appositions'' alongside tight ...
communication with cardiac
myocyte
A muscle cell, also known as a myocyte, is a mature contractile Cell (biology), cell in the muscle of an animal. In humans and other vertebrates there are three types: skeletal muscle, skeletal, smooth muscle, smooth, and Cardiac muscle, cardiac ...
s.
Macrophages can be classified on basis of the fundamental function and activation. According to this grouping, there are
classically activated (M1) macrophages, wound-healing macrophages (also known as
alternatively-activated (M2) macrophages), and
regulatory macrophages (Mregs).
Development
Macrophages that reside in adult healthy tissues either derive from circulating
monocytes
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also i ...
or are established before birth and then maintained during adult life independently of monocytes. By contrast, most of the macrophages that accumulate at diseased sites typically derive from circulating monocytes.
Leukocyte extravasation
In immunology, leukocyte extravasation (also commonly known as leukocyte adhesion cascade or diapedesis ŌĆō the passage of cells through the intact vessel wall) is the movement of leukocytes (white blood cells) out of the circulatory system (ext ...
describes
monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
entry into damaged tissue through the
endothelium
The endothelium (: endothelia) is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the r ...
of
blood vessels
Blood vessels are the tubular structures of a circulatory system that transport blood throughout many animalsŌĆÖ bodies. Blood vessels transport blood cells, nutrients, and oxygen to most of the tissues of a body. They also take waste an ...
as they become macrophages. Monocytes are attracted to a damaged site by chemical substances through
chemotaxis
Chemotaxis (from ''chemical substance, chemo-'' + ''taxis'') is the movement of an organism or entity in response to a chemical stimulus. Somatic cells, bacteria, and other single-cell organism, single-cell or multicellular organisms direct thei ...
, triggered by a range of stimuli including damaged cells, pathogens and
cytokines
Cytokines () are a broad and loose category of small proteins (~5ŌĆō25 kDa) important in cell signaling.
Cytokines are produced by a broad range of cells, including immune cells like macrophages, B cell, B lymphocytes, T cell, T lymphocytes ...
released by macrophages already at the site. At some sites such as the testis, macrophages have been shown to populate the organ through proliferation. Unlike short-lived
neutrophils
Neutrophils are a type of phagocytic white blood cell and part of innate immunity. More specifically, they form the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. Their functions vary in different ...
, macrophages survive longer in the body, up to several months.
Function

Phagocytosis
Macrophages are
professional phagocytes and are highly specialized in removal of dying or dead cells and cellular debris. This role is important in chronic inflammation, as the early stages of inflammation are dominated by neutrophils, which expend themselves and are ingested by macrophages.
Macrophages normally present themselves at the wound site within 2 days following the injury.
The neutrophils are at first attracted to a site, where they perform their function and die, before they or their
neutrophil extracellular traps
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens. Neutrophils are the immune system's first line of defense against infection and have conventionally bee ...
are phagocytized by the macrophages.
[ The first wave of neutrophils acts for approximately 2 days at the site and signals to attract macrophages. These macrophages will then ingest the aged neutrophils.]neural tissue
Nervous tissue, also called neural tissue, is the main tissue component of the nervous system. The nervous system regulates and controls body functions and activity. It consists of two parts: the central nervous system (CNS) comprising the brain ...
, bone, spleen and connective tissue, ingesting foreign materials such as pathogens and recruiting additional macrophages if needed. The phagocytosis and clearance of apoptotic remains is called efferocytosis
In cell biology, efferocytosis (from ''efferre'', Latin for 'to carry out' (to the grave), extended meaning 'to bury') is the process by which apoptotic cells are removed by phagocytic cells. It can be regarded as the 'burying of dead cells'.
Du ...
and is also carried out by other cell types, not all of which are professional phagocytes.
When a macrophage ingests a pathogen, the pathogen becomes trapped in a phagosome
In cell biology, a phagosome is a vesicle formed around a particle engulfed by a phagocyte via phagocytosis. Professional phagocytes include macrophages, neutrophils, and dendritic cells (DCs).
A phagosome is formed by the fusion of the cel ...
, which then fuses with a lysosome
A lysosome () is a membrane-bound organelle that is found in all mammalian cells, with the exception of red blood cells (erythrocytes). There are normally hundreds of lysosomes in the cytosol, where they function as the cellŌĆÖs degradation cent ...
. Within the phagolysosome
In biology, a phagolysosome, or endolysosome, is a cytoplasmic body formed by the fusion of a phagosome with a lysosome in a process that occurs during phagocytosis. Formation of phagolysosomes is essential for the intracellular destruction of mic ...
, enzymes
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as pro ...
and toxic peroxides digest the pathogen. However, some bacteria (such as ''Mycobacterium tuberculosis
''Mycobacterium tuberculosis'' (M. tb), also known as Koch's bacillus, is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis.
First discovered in 1882 by Robert Koch, ''M. tuberculosis'' ha ...
)'' have become resistant to these methods of digestion. Typhoidal ''Salmonellae'' induce their own phagocytosis by host macrophages in vivo and inhibit digestion by lysosomal action, thereby using macrophages for their own replication and causing macrophage apoptosis. Macrophages are capable of engulfing and digesting many bacteria during their life. They can die eventually due to factors including pathogenic cytotoxicity, oxidative stress, and phagocytosis-induced apoptosis. Phagocytosis-induced apoptosis results from the powerful apoptotic stimulus of consuming bacteria and is observed in (at least) macrophages and neutrophils.
Role in innate immune response
When a pathogen invades, tissue resident macrophages are among the first cells to respond.
Phagocytosis of pathogens
 Macrophages can internalize antigens through receptor-mediated phagocytosis.
Macrophages can internalize antigens through receptor-mediated phagocytosis.pattern recognition receptor
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of th ...
s (PRRs) that can recognize microbe-associated molecular patterns
Pathogen-associated molecular patterns (PAMPs) are small molecular motifs conserved within a class of microbes, but not present in the host. They are recognized by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in both ...
(MAMPs) from pathogens. Many PRRs, such as toll-like receptor
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane protein, single-spanning receptor (biochemistry), receptors usually expressed on sentinel cells such as macrophages ...
s (TLRs), scavenger receptors Scavenger receptor may refer to:
*Scavenger receptor (immunology)
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein, with the defining prope ...
(SRs), C-type lectin receptors, among others, recognize pathogens for phagocytosis.opsonin
Opsonins are extracellular proteins that, when bound to substances or cells, induce phagocytes to phagocytose the substances or cells with the opsonins bound. Thus, opsonins act as tags to label things in the body that should be phagocytosed (i.e. ...
s, which are molecules that attach to pathogens and mark them for phagocytosis.complement proteins
The complement system, also known as complement cascade, is a part of the humoral, innate immune system and enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote in ...
and antibodies can bind to antigens and opsonize them. Macrophages have complement receptor 1 (CR1) and 3 (CR3) that recognize pathogen-bound complement proteins C3b and iC3b, respectively, as well as fragment crystallizable ╬│ receptors (Fc╬│Rs) that recognize the fragment crystallizable (Fc) region of antigen-bound immunoglobulin G
Immunoglobulin G (IgG) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG ...
(IgG) antibodies.respiratory burst
Respiratory burst (or oxidative burst) is the rapid release of the reactive oxygen species (ROS), superoxide anion () and hydrogen peroxide (), from different cell types.
This is usually utilised for mammalian immunological defence, but also pl ...
where more oxygen is consumed to supply the energy required for producing reactive oxygen species (ROS) and other antimicrobial molecules that digest the consumed pathogens.
Chemical secretion
Recognition of MAMPs by PRRs can activate tissue resident macrophages to secrete proinflammatory cytokines that recruit other immune cells. Among the PRRs, TLRs play a major role in signal transduction leading to cytokine production.NF-╬║B
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-╬║B) is a family of transcription factor protein complexes that controls transcription (genetics), transcription of DNA, cytokine production and cell survival. NF-╬║B is found i ...
and results in transcription of the genes for several proinflammatory cytokines, including IL-1╬▓
Interleukin-1 beta (IL-1╬▓) also known as leukocytic pyrogen, leukocytic endogenous mediator, mononuclear cell factor, lymphocyte activating factor and other names, is a cytokine protein that in humans is encoded by the ''IL1B'' gene."Catabolin" ...
, IL-6, TNF-╬▒
Tumor necrosis factor (TNF), formerly known as TNF-╬▒, is a chemical messenger produced by the immune system that induces inflammation. TNF is produced primarily by activated macrophages, and induces inflammation by binding to its receptors o ...
, IL-12B, and type I interferons such as IFN-╬▒ and IFN-╬▓. Systemically, IL-1╬▓, IL-6, and TNF-╬▒ induce fever and initiate the acute phase response in which the liver secretes acute phase proteins
Acute-phase proteins (APPs) are a class of proteins whose concentrations in blood plasma either increase (positive acute-phase proteins) or decrease (negative acute-phase proteins) in response to inflammation. This response is called the ''acute-p ...
.leukocyte extravasation
In immunology, leukocyte extravasation (also commonly known as leukocyte adhesion cascade or diapedesis ŌĆō the passage of cells through the intact vessel wall) is the movement of leukocytes (white blood cells) out of the circulatory system (ext ...
.prostaglandin
Prostaglandins (PG) are a group of physiology, physiologically active lipid compounds called eicosanoids that have diverse hormone-like effects in animals. Prostaglandins have been found in almost every Tissue (biology), tissue in humans and ot ...
s (PGs) which are important mediators of inflammation and pain. Among the PGs, anti-inflammatory PGE2
Prostaglandin E2 (PGE2), also known as dinoprostone, is a naturally occurring prostaglandin with oxytocic properties that is used as a medication. Dinoprostone is used in labor induction, bleeding after delivery, termination of pregnancy, ...
and pro-inflammatory PGD2
Prostaglandin D2 (or PGD2) is a prostaglandin that binds to the receptor PTGDR (DP1), as well as CRTH2 (DP2). It is a major prostaglandin produced by mast cells ŌĆō recruits Th2 cells, eosinophils, and basophils. In mammalian organs, large a ...
increase the most after activation, with PGE2 increasing expression of IL-10 and inhibiting production of TNFs via the COX-2
Cyclooxygenase-2 (COX-2), also known as prostaglandin-endoperoxide synthase 2 ( HUGO PTGS2), is an enzyme that in humans is encoded by the ''PTGS2'' gene. In humans it is one of three cyclooxygenases. It is involved in the conversion of arachid ...
pathway.
Neutrophil
Neutrophils are a type of phagocytic white blood cell and part of innate immunity. More specifically, they form the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. Their functions vary in differe ...
s are among the first immune cells recruited by macrophages to exit the blood via extravasation and arrive at the infection site.chemokine
Chemokines (), or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In addit ...
s such as CXCL1
The chemokine (C-X-C motif) ligand 1 (CXCL1) is a small peptide belonging to the CXC chemokine family that acts as a chemoattractant for several immune cells, especially neutrophils or other non-hematopoietic cells to the site of injury or infecti ...
, CXCL2
Chemokine (C-X-C motif) ligand 2 (CXCL2) is a small cytokine belonging to the CXC chemokine family that is also called ''macrophage inflammatory protein 2-alpha'' (MIP2-alpha), ''Growth-regulated protein beta'' (Gro-beta) and ''Gro oncogene-2'' ...
, and CXCL8 (IL-8) that attract neutrophils to the site of infection.CCL2
The chemokine (C-C motif) ligand 2 (CCL2) is also referred to as monocyte chemoattractant protein 1 (MCP1) and small inducible cytokine A2. CCL2 is a small cytokine that belongs to the CC chemokine family. CCL2 tightly regulates cellular mecha ...
, CCL4
Chemokine (C-C motif) ligands 4 (also CCL4) previously known as macrophage inflammatory protein (MIP-1╬▓), is a protein which in humans is encoded by the ''CCL4'' gene. ''CCL4'' belongs to a cluster of genes located on 17q11-q21 of the chromoso ...
, CCL5
Chemokine (C-C motif) ligand 5 (also CCL5) is a protein which in humans is encoded by the ''CCL5'' gene. The gene has been discovered in 1990 by ''in situ'' hybridisation and it is localised on 17q11.2-q12 chromosome.
It is also known as RANTES ...
, CXCL8
Interleukin 8 (IL-8 or chemokine (C-X-C motif) ligand 8, CXCL8) is a chemokine produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells. Endothelial cells store IL-8 in their storage ...
, CXCL9
Chemokine (C-X-C motif) ligand 9 (CXCL9) is a small cytokine belonging to the CXC chemokine family that is also known as monokine induced by gamma interferon (MIG). The CXCL9 is one of the chemokine which plays role to induce chemotaxis, promote ...
, CXCL10
C-X-C motif chemokine ligand 10 (CXCL10) also known as Interferon gamma-induced protein 10 (IP-10) or small-inducible cytokine B10 is an 8.7 kDa protein that in humans is encoded by the ''CXCL10'' gene. C-X-C motif chemokine 10 is a small cytokin ...
, and CXCL11
C-X-C motif chemokine 11 (CXCL11) is a protein that in humans is encoded by the ''CXCL11'' gene.
C-X-C motif chemokine 11 is a small cytokine belonging to the CXC chemokine family that is also called ''Interferon-inducible T-cell alpha chemoattra ...
.interferon gamma
Interferon gamma (IFNG or IFN-╬│) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. ...
(IFN-╬│) by NK cells, which serves as an important source of IFN-╬│ before the adaptive immune system is activated.CCL5
Chemokine (C-C motif) ligand 5 (also CCL5) is a protein which in humans is encoded by the ''CCL5'' gene. The gene has been discovered in 1990 by ''in situ'' hybridisation and it is localised on 17q11.2-q12 chromosome.
It is also known as RANTES ...
, CXCL9
Chemokine (C-X-C motif) ligand 9 (CXCL9) is a small cytokine belonging to the CXC chemokine family that is also known as monokine induced by gamma interferon (MIG). The CXCL9 is one of the chemokine which plays role to induce chemotaxis, promote ...
, CXCL10
C-X-C motif chemokine ligand 10 (CXCL10) also known as Interferon gamma-induced protein 10 (IP-10) or small-inducible cytokine B10 is an 8.7 kDa protein that in humans is encoded by the ''CXCL10'' gene. C-X-C motif chemokine 10 is a small cytokin ...
, and CXCL11
C-X-C motif chemokine 11 (CXCL11) is a protein that in humans is encoded by the ''CXCL11'' gene.
C-X-C motif chemokine 11 is a small cytokine belonging to the CXC chemokine family that is also called ''Interferon-inducible T-cell alpha chemoattra ...
.
Role in adaptive immunity

Interactions with CD4+ T Helper Cells
Macrophages are professional antigen presenting cells (APC), meaning they can present peptides from phagocytosed antigens on major histocompatibility complex (MHC) II molecules on their cell surface for T helper cells.T cell receptor
The T-cell receptor (TCR) is a protein complex, located on the surface of T cells (also called T lymphocytes). They are responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. ...
(TCR), and 2) recognition of pathogens by PRRs induce macrophages to upregulate the co-stimulatory molecules CD80
The Cluster of differentiation 80 (also CD80 and B7-1) is a B7, type I membrane protein in the immunoglobulin superfamily, with an extracellular immunoglobulin constant-like domain and a variable-like domain required for receptor binding. It is c ...
and CD86
Cluster of Differentiation 86 (also known as CD86 and B7-2) is a protein constitutively expressed on dendritic cells, Langerhans cells, macrophages, B-cells (including memory B-cells), and on other antigen-presenting cells. Along with CD80, CD ...
(also known as B7) that binds to CD28
CD28 (Cluster of Differentiation 28) is a protein expressed on T cells that provides essential co-stimulation, co-stimulatory signals required for T cell activation and survival. When T cells are stimulated through CD28 in conjunction with the T- ...
on T helper cells to supply the co-stimulatory signal.IL-2
The Ilyushin Il-2 (Russian language, Russian: ąśą╗čīčÄ╠üčłąĖąĮ ąśą╗-2) is a Ground attack aircraft, ground-attack plane that was produced by the Soviet Union in large numbers during the World War II, Second World War. The word ''shturmov├Łk'' (C ...
signaling in T cells upregulates the expression of anti-apoptotic protein Bcl-2
Bcl-2, encoded in humans by the ''BCL2'' gene, is the founding member of the Bcl-2 family of regulator proteins. BCL2 blocks programmed cell death (apoptosis) while other BCL2 family members can either inhibit or induce it. It was the first a ...
, but T cell production of IL-2 and the high-affinity IL-2 receptor IL-2RA both require continued signal from TCR recognition of MHC-bound antigen.
Activation
Macrophages can achieve different activation phenotypes through interactions with different subsets of T helper cells, such as TH1 and TH2.
= Classical
=
TH1 cells play an important role in classical macrophage activation as part of type 1 immune response against intracellular pathogens (such as intracellular bacteria
Intracellular bacteria are bacteria that have the capability to enter and survive within the cells of the host organism. These bacteria include many different pathogens that live in the cytoplasm and nuclei of the host cell's they inhabit. ''Myco ...
) that can survive and replicate inside host cells, especially those pathogens that replicate even after being phagocytosed by macrophages. After the TCR of TH1 cells recognize specific antigen peptide-bound MHC class II molecules on macrophages, TH1 cells 1) secrete IFN-╬│ and 2) upregulate the expression of CD40 ligand
CD154, also called CD40 ligand or CD40L, is a protein that is primarily expressed on activated T cells and is a member of the TNF superfamily of molecules. It binds to CD40 on antigen-presenting cells (APC), which leads to many effects dependin ...
(CD40L), which binds to CD40
Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on T helper cell, TH cells to CD40 activates antigen presenting cells and i ...
on macrophages.nitric oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes den ...
(NO) and superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
(O2-).Fas ligand
Fas ligand (FasL, also known as CD95L or Apo-1L) is a type-II transmembrane protein in the tumor necrosis factor (TNF) superfamily. It binds to the Fas receptor (CD95) to induce apoptosis, and also activates non-apoptotic pathways such as NF-╬║B ...
(FasL) and lymphotoxin beta
Lymphotoxin-beta (LT-beta) formerly known as tumor necrosis factor C (TNF-C) is a protein that in humans is encoded by the ''LTB'' gene.
Function
Lymphotoxin beta is a type II membrane protein of the TNF family. It anchors lymphotoxin-alpha ...
(LT-╬▓) to help kill chronically infected macrophages that can no longer kill pathogens.TNF-╬▒
Tumor necrosis factor (TNF), formerly known as TNF-╬▒, is a chemical messenger produced by the immune system that induces inflammation. TNF is produced primarily by activated macrophages, and induces inflammation by binding to its receptors o ...
and LT-╬▒ to make blood vessels easier for monocytes to bind to and exit.CCL2
The chemokine (C-C motif) ligand 2 (CCL2) is also referred to as monocyte chemoattractant protein 1 (MCP1) and small inducible cytokine A2. CCL2 is a small cytokine that belongs to the CC chemokine family. CCL2 tightly regulates cellular mecha ...
as a chemoattractant for monocytes. IL-3 and GM-CSF released by TH1 cells stimulate more monocyte production in the bone marrow.Mycobacterium tuberculosis
''Mycobacterium tuberculosis'' (M. tb), also known as Koch's bacillus, is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis.
First discovered in 1882 by Robert Koch, ''M. tuberculosis'' ha ...
'', the pathogen is contained through the formation of granuloma
A granuloma is an aggregation of macrophages (along with other cells) that forms in response to chronic inflammation. This occurs when the immune system attempts to isolate foreign substances that it is otherwise unable to eliminate. Such sub ...
, an aggregation of infected macrophages surrounded by activated T cells.
= Alternative
=
TH2 cells play an important role in alternative macrophage activation as part of type 2 immune response against large extracellular pathogens like helminths
Parasitic worms, also known as helminths, are a polyphyletic group of large macroparasites; adults can generally be seen with the naked eye. Many are intestinal worms that are soil-transmitted and infect the gastrointestinal tract. Other par ...
.arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidinium, guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (ŌłÆCO2ŌłÆ) a ...
to ornithine
Ornithine is a non-proteinogenic ╬▒-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of th ...
and urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (ŌĆō) joined by a carbonyl functional group (ŌĆōC(=O)ŌĆō). It is thus the simplest am ...
.proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the p ...
, which is essential for synthesizing collagen
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a trip ...
.
Interactions with CD8+ cytotoxic t cells
Another part of the adaptive immunity activation involves stimulating CD8+ via cross presentation of antigens peptides on MHC class I molecules. Studies have shown that proinflammatory macrophages are capable of cross presentation of antigens on MHC class I molecules, but whether macrophage cross-presentation plays a role in naïve or memory CD8+ T cell activation is still unclear.
Interactions with B cells
Macrophages have been shown to secrete cytokines BAFF and APRIL, which are important for plasma cell isotype switching. APRIL and IL-6 secreted by macrophage precursors in the bone marrow help maintain survival of plasma cells homed to the bone marrow.
Subtypes
There are several activated forms of macrophages.IFN-gamma
Interferon gamma (IFNG or IFN-╬│) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. ...
, and secrete high levels of IL-12 and low levels of IL-10. M1 macrophages have pro-inflammatory, bactericidal, and phagocytic functions.TGF-beta
Transforming growth factor beta (TGF-╬▓) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-╬▓ 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other ...
and low levels of IL-12. Tumor-associated macrophages are mainly of the M2 phenotype, and seem to actively promote tumor growth.
Macrophages exist in a variety of phenotypes which are determined by the role they play in wound maturation. Phenotypes can be predominantly separated into two major categories; M1 and M2. M1 macrophages are the dominating phenotype observed in the early stages of inflammation and are activated by four key mediators: interferon-╬│ (IFN-╬│), tumor necrosis factor (TNF), and damage associated molecular patterns (DAMPs). These mediator molecules create a pro-inflammatory response that in return produce pro-inflammatory cytokines like Interleukin-6 and TNF. Unlike M1 macrophages, M2 macrophages secrete an anti-inflammatory response via the addition of Interleukin-4 or Interleukin-13. They also play a role in wound healing and are needed for revascularization and reepithelialization. M2 macrophages are divided into four major types based on their roles: M2a, M2b, M2c, and M2d. How M2 phenotypes are determined is still up for discussion but studies have shown that their environment allows them to adjust to whichever phenotype is most appropriate to efficiently heal the wound.[ There is a phenotype shift from M1 to M2 macrophages in acute wounds, however this shift is impaired for chronic wounds. This dysregulation results in insufficient M2 macrophages and its corresponding growth factors that aid in wound repair. With a lack of these growth factors/anti-inflammatory cytokines and an overabundance of pro-inflammatory cytokines from M1 macrophages chronic wounds are unable to heal in a timely manner. Normally, after neutrophils eat debris/pathogens they perform apoptosis and are removed. At this point, inflammation is not needed and M1 undergoes a switch to M2 (anti-inflammatory). However, dysregulation occurs as the M1 macrophages are unable/do not phagocytose neutrophils that have undergone apoptosis leading to increased macrophage migration and inflammation.][
Both M1 and M2 macrophages play a role in promotion of ]atherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis, characterized by development of abnormalities called lesions in walls of arteries. This is a chronic inflammatory disease involving many different cell types and is driven by eleva ...
. M1 macrophages promote atherosclerosis by inflammation. M2 macrophages can remove cholesterol from blood vessels, but when the cholesterol is oxidized, the M2 macrophages become apoptotic
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemical events lead to characteristic cell changes ( morphology) and death. These ...
foam cells contributing to the atheromatous plaque
An atheroma, or atheromatous plaque, is an abnormal accumulation of material in the inner layer of an arterial wall.
The material consists of mostly macrophage cells, or debris, containing lipids, calcium and a variable amount of fibrous conne ...
of atherosclerosis.
Role in muscle regeneration
The first step to understanding the importance of macrophages in muscle repair, growth, and regeneration is that there are two "waves" of macrophages with the onset of damageable muscle useŌĆō subpopulations that do and do not directly have an influence on repairing muscle. The initial wave is a phagocytic population that comes along during periods of increased muscle use that are sufficient to cause muscle membrane lysis and membrane inflammation, which can enter and degrade the contents of injured muscle fibers.
Role in wound healing
Macrophages are essential for wound healing
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.
In undamaged skin, the epidermis (surface, epithelial layer) and dermis (deeper, connective layer) form a protective barrier again ...
.[de la Torre J., Sholar A. (2006)]
Wound healing: Chronic wounds
Emedicine.com. Accessed 20 January 2008. They replace polymorphonuclear neutrophil
Neutrophils are a type of phagocytic white blood cell and part of innate immunity. More specifically, they form the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. Their functions vary in different ...
s as the predominant cells in the wound by day two after injury.monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
s from the bloodstream enter the area through blood vessel walls.spleen
The spleen (, from Ancient Greek '' ŽāŽĆ╬╗╬«╬Į'', splßĖŚn) is an organ (biology), organ found in almost all vertebrates. Similar in structure to a large lymph node, it acts primarily as a blood filter.
The spleen plays important roles in reg ...
contains half the body's monocytes in reserve ready to be deployed to injured tissue.debride
Debridement is the medical removal of dead, damaged, or infected tissue to improve the healing potential of the remaining healthy tissue. Removal may be surgical, mechanical, chemical, autolytic (self-digestion), or by maggot therapy.
In p ...
damaged tissue by releasing proteases.[Rosenberg L., de la Torre J. (2006)]
Wound Healing, Growth Factors
Emedicine.com. Accessed 20 January 2008. Macrophages may also restrain the contraction phase.oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
content of their surroundings to produce factors that induce and speed angiogenesis
Role in limb regeneration
Scientists have elucidated that as well as eating up material debris, macrophages are involved in the typical limb regeneration in the salamander.[
]
Role in iron homeostasis
As described above, macrophages play a key role in removing dying or dead cells and cellular debris. Red blood cell, Erythrocytes have a lifespan on average of 120 days and so are constantly being destroyed by macrophages in the spleen and liver. Macrophages will also engulf macromolecules, and so play a key role in the pharmacokinetics of parenteral irons.
The iron that is released from the haemoglobin is either stored internally in ferritin or is released into the circulation via ferroportin. In cases where systemic iron levels are raised, or where inflammation is present, raised levels of hepcidin act on macrophage ferroportin channels, leading to iron remaining within the macrophages.
Role in pigment retainment
 Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such as tattoos), from extracellular space. In contrast to dendritic juncional melanocytes, which Melanin, synthesize melanosomes and contain various stages of their development, the melanophages only accumulate Phagocytosis, phagocytosed melanin in lysosome-like phagosomes. This occurs repeatedly as the pigment from dead dermal macrophages is phagocytosed by their successors, preserving the tattoo in the same place.
Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such as tattoos), from extracellular space. In contrast to dendritic juncional melanocytes, which Melanin, synthesize melanosomes and contain various stages of their development, the melanophages only accumulate Phagocytosis, phagocytosed melanin in lysosome-like phagosomes. This occurs repeatedly as the pigment from dead dermal macrophages is phagocytosed by their successors, preserving the tattoo in the same place.
Role in tissue homeostasis
Every tissue harbors its own specialized population of resident macrophages, which entertain reciprocal interconnections with the stroma and functional tissue. These resident macrophages are sessile (non-migratory), provide essential growth factors to support the physiological function of the tissue (e.g. macrophage-neuronal crosstalk in the guts), and can actively protect the tissue from inflammatory damage.
Nerve-associated macrophages
Nerve-associated macrophages or NAMs are those tissue-resident macrophages that are associated with nerves. Some of them are known to have an elongated morphology of up to 200╬╝m
Clinical significance
Due to their role in phagocytosis, macrophages are involved in many diseases of the immune system. For example, they participate in the formation of granuloma
A granuloma is an aggregation of macrophages (along with other cells) that forms in response to chronic inflammation. This occurs when the immune system attempts to isolate foreign substances that it is otherwise unable to eliminate. Such sub ...
s, inflammatory lesions that may be caused by a large number of diseases. Some disorders, mostly rare, of ineffective phagocytosis and macrophage function have been described, for example.
As a host for intracellular pathogens
In their role as a phagocytic immune cell macrophages are responsible for engulfing pathogens to destroy them. Some pathogens subvert this process and instead live inside the macrophage. This provides an environment in which the pathogen is hidden from the immune system and allows it to replicate.
Diseases with this type of behaviour include tuberculosis (caused by ''Mycobacterium tuberculosis
''Mycobacterium tuberculosis'' (M. tb), also known as Koch's bacillus, is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis.
First discovered in 1882 by Robert Koch, ''M. tuberculosis'' ha ...
'') and leishmaniasis (caused by ''Leishmania'' species).
In order to minimize the possibility of becoming the host of an intracellular bacteria, macrophages have evolved defense mechanisms such as induction of nitric oxide and reactive oxygen intermediates, which are toxic to microbes. Macrophages have also evolved the ability to restrict the microbe's nutrient supply and induce autophagy.
Tuberculosis
Once engulfed by a macrophage, the causative agent of tuberculosis, ''Mycobacterium tuberculosis'',
Leishmaniasis
Upon phagocytosis by a macrophage, the ''Leishmania'' parasite finds itself in a phagocytic vacuole. Under normal circumstances, this phagocytic vacuole would develop into a lysosome and its contents would be digested. ''Leishmania'' alter this process and avoid being destroyed; instead, they make a home inside the vacuole.
Chikungunya
Infection of macrophages in joints is associated with local inflammation during and after the acute phase of ''Chikungunya'' (caused by CHIKV or Chikungunya virus).
Others
Adenovirus (most common cause of pink eye) can remain latent in a host macrophage, with continued viral shedding 6ŌĆō18 months after initial infection.
''Brucella spp.'' can remain latent in a macrophage via inhibition of phagosome
In cell biology, a phagosome is a vesicle formed around a particle engulfed by a phagocyte via phagocytosis. Professional phagocytes include macrophages, neutrophils, and dendritic cells (DCs).
A phagosome is formed by the fusion of the cel ...
ŌĆōlysosome
A lysosome () is a membrane-bound organelle that is found in all mammalian cells, with the exception of red blood cells (erythrocytes). There are normally hundreds of lysosomes in the cytosol, where they function as the cellŌĆÖs degradation cent ...
fusion; causes brucellosis (undulant fever).
''Legionella pneumophila'', the causative agent of Legionnaires' disease, also establishes residence within macrophages.
Heart disease
Macrophages are the predominant cells involved in creating the progressive plaque lesions of atherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis, characterized by development of abnormalities called lesions in walls of arteries. This is a chronic inflammatory disease involving many different cell types and is driven by eleva ...
.
Focal recruitment of macrophages occurs after the onset of acute myocardial infarction. These macrophages function to remove debris, apoptotic cells and to prepare for Regeneration (biology), tissue regeneration. Macrophages protect against ischemia-induced ventricular tachycardia in hypokalemic mice.
HIV infection
Macrophages also play a role in human Immunodeficiency Virus, human immunodeficiency virus (HIV) infection. Like T cells, macrophages can be infected with HIV, and even become a reservoir of ongoing virus replication throughout the body. HIV can enter the macrophage through binding of gp120 to CD4 and second membrane receptor, CCR5 (a chemokine receptor). Both circulating monocytes and macrophages serve as a reservoir for the virus. Macrophages are better able to resist infection by HIV-1 than CD4+ T cells, although susceptibility to HIV infection differs among macrophage subtypes.
Cancer
Macrophages can contribute to tumor growth and progression by promoting tumor cell proliferation and invasion, fostering tumor angiogenesis and suppressing antitumor immune cells.
Cancer therapy
Experimental studies indicate that macrophages can affect all therapeutic modalities, including surgery, chemotherapy, radiotherapy, immunotherapy and targeted therapy.phagocytosis
Phagocytosis () is the process by which a cell (biology), cell uses its plasma membrane to engulf a large particle (Ōēź 0.5 ╬╝m), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs ph ...
) following treatments that kill these cells; they can serve as drug depots for some anticancer drugs; they can also be activated by some therapies to promote antitumor immunity. Macrophages can also be deleterious in several ways: for example they can suppress various chemotherapies, radiotherapies and immunotherapies. Because macrophages can regulate tumor progression, therapeutic strategies to reduce the number of these cells, or to manipulate their phenotypes, are currently being tested in cancer patients. However, macrophages are also involved in antibody mediated cytotoxicity (ADCC) and this mechanism has been proposed to be important for certain cancer immunotherapy antibodies. Similarly, studies identified macrophages genetically engineered to express chimeric antigen receptors as promising therapeutic approach to lowering tumor burden.
Obesity
It has been observed that increased number of pro-inflammatory macrophages within obese adipose tissue contributes to obesity complications including insulin resistance and diabetes type 2.
The modulation of the inflammatory state of adipose tissue macrophages has therefore┬Ābeen considered a possible therapeutic target to treat obesity-related diseases. Although adipose tissue macrophages are subject to anti-inflammatory homeostatic control by sympathetic innervation, experiments using Beta-2 adrenergic receptor, ADRB2 gene knockout mice indicate that this effect is indirectly exerted through the modulation of adipocyte function, and not through direct Beta-2 adrenergic receptor activation, suggesting that adrenergic stimulation of macrophages may be insufficient to impact adipose tissue inflammation or function in obesity.
Within the fat (Adipose tissue, adipose) tissue of CCR2 deficient Mouse, mice, there is an increased number of eosinophils, greater alternative macrophage activation, and a propensity towards type 2 cytokine expression. Furthermore, this effect was exaggerated when the mice became Obesity, obese from a high fat diet. This is partially caused by a phenotype switch of macrophages induced by necrosis of fat cells (adipocytes). In an obese individual some adipocytes burst and undergo necrotic death, which causes the residential M2 macrophages to switch to M1 phenotype. This is one of the causes of a low-grade systemic chronic inflammatory state associated with obesity.
Intestinal macrophages
Though very similar in structure to tissue macrophages, intestinal macrophages have evolved specific characteristics and functions given their natural environment, which is in the digestive tract. Macrophages and intestinal macrophages have high plasticity causing their phenotype to be altered by their environments. Like macrophages, intestinal macrophages are differentiated monocytes, though intestinal macrophages have to coexist with the Microbiota, microbiome in the intestines. This is a challenge considering the bacteria found in the gut are not recognized as "self" and could be potential targets for phagocytosis by the macrophage.
Role in disease
Intestinal macrophages have been shown to play a role in inflammatory bowel disease (IBD), such as Crohn's disease (CD) and ulcerative colitis (UC). In a healthy gut, intestinal macrophages limit the inflammatory response in the gut, but in a disease-state, intestinal macrophage numbers and diversity are altered. This leads to inflammation of the gut and disease symptoms of IBD. Intestinal macrophages are critical in maintaining gut homeostasis. The presence of inflammation or pathogen alters this homeostasis, and concurrently alters the intestinal macrophages. There has yet to be a determined mechanism for the alteration of the intestinal macrophages by recruitment of new monocytes or changes in the already present intestinal macrophages.
Media
File:S4-J774 Cells with Conidia in Liquid Media.ogg, An active J774 macrophage is seen taking up four conidia in a co-operative manner. The J774 cells were treated with 5ng/ml interferon-╬│ one night before filming with conidia. Observations were made every 30s over a 2.5hr period.
File:S3-Alveolar Macrophages with Conidia in Liquid Medium.ogv, Two highly active alveolar macrophage
An alveolar macrophage, pulmonary macrophage, (or dust cell, or dust eater) is a type of macrophage, a phagocytosis#Professional phagocytic cells, professional phagocyte, found in the airways and at the level of the pulmonary alveolus, alveoli in ...
s can be seen ingesting conidia. Time lapse is 30s per frame over 2.5hr.
History
Macrophages were first discovered late in the 19th century by zoologist ├ēlie Metchnikoff
Ilya Ilyich Mechnikov (; ŌĆō 15 July 1916), also spelled ├ēlie Metchnikoff, was a zoologist from the Russian Empire of Moldavian noble ancestry and alshereat archive.org best known for his research in immunology (study of immune systems) and ...
. Metchnikoff revolutionized the branch of macrophages by combining philosophical insights and the evolutionary study of life. Later on, Van Furth during the 1960s proposed the idea that circulating blood monocytes in adults allowed for the origin of all tissue macrophages. In recent years, publishing regarding macrophages has led people to believe that multiple resident tissue macrophages are independent of the blood monocytes as it is formed during the embryonic stage of development. Within the 21st century, all the ideas concerning the origin of macrophages (present in tissues) were compiled together to suggest that physiologically complex organisms, from macrophages independently by mechanisms that don't have to depend on the blood monocytes.
See also
* Bacteriophage
* Dendritic cell
* Histiocyte
* List of distinct cell types in the adult human body
References
{{Authority control
Macrophages,
Phagocytes
Cell biology
Immune system
Human cells
Articles containing video clips
Connective tissue cells
Lymphatic system
 A majority of macrophages are stationed at strategic points where microbial invasion or accumulation of foreign particles is likely to occur. These cells together as a group are known as the
A majority of macrophages are stationed at strategic points where microbial invasion or accumulation of foreign particles is likely to occur. These cells together as a group are known as the  Macrophages can internalize antigens through receptor-mediated phagocytosis. Macrophages have a wide variety of
Macrophages can internalize antigens through receptor-mediated phagocytosis. Macrophages have a wide variety of 
 Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such as tattoos), from extracellular space. In contrast to dendritic juncional melanocytes, which Melanin, synthesize melanosomes and contain various stages of their development, the melanophages only accumulate Phagocytosis, phagocytosed melanin in lysosome-like phagosomes. This occurs repeatedly as the pigment from dead dermal macrophages is phagocytosed by their successors, preserving the tattoo in the same place.
Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such as tattoos), from extracellular space. In contrast to dendritic juncional melanocytes, which Melanin, synthesize melanosomes and contain various stages of their development, the melanophages only accumulate Phagocytosis, phagocytosed melanin in lysosome-like phagosomes. This occurs repeatedly as the pigment from dead dermal macrophages is phagocytosed by their successors, preserving the tattoo in the same place.