Leghemoglobin on:
[Wikipedia]
[Google]
[Amazon]
 Leghemoglobin (also leghaemoglobin or legoglobin) is an oxygen-carrying phytoglobin found in the
Leghemoglobin (also leghaemoglobin or legoglobin) is an oxygen-carrying phytoglobin found in the
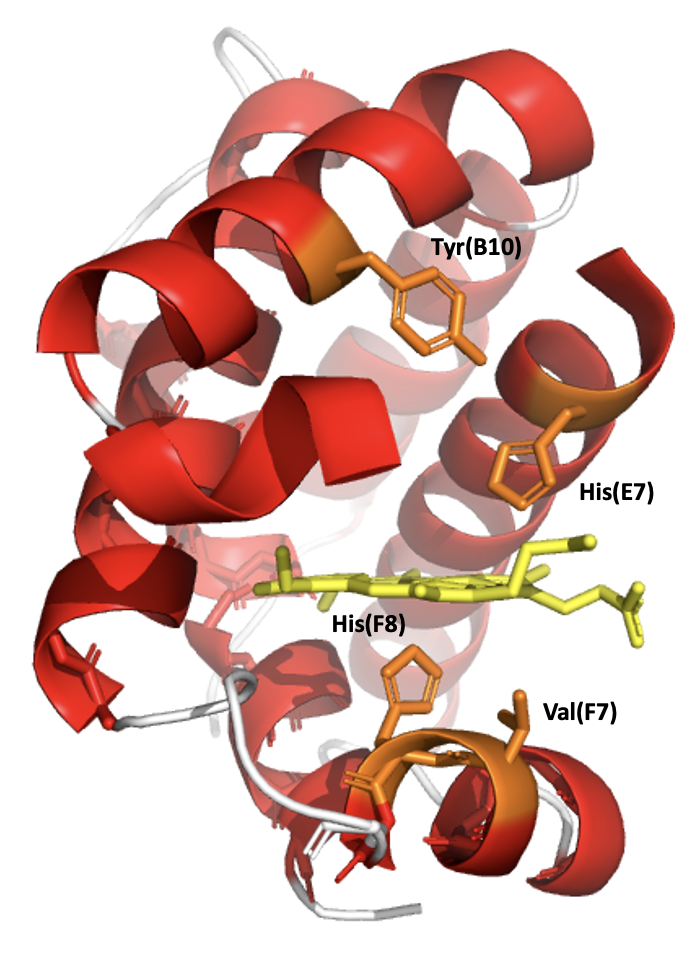 Oxygen binding affinities of leghemoglobins are between 11 and 24 times higher than oxygen binding affinities of sperm whale myoglobin. Differences in the affinities are due to differential rates of association between the two types of proteins. One explanation of this phenomenon is that in myoglobin, a bound water molecule is stabilized in a pocket surrounding the heme group. This water group must be displaced in order for oxygen to bind. No such water is bound in the analogous pocket of leghemoglobin, so it is easier for an oxygen molecule to approach the leghemoglobin heme. Leghemoglobin has a slow oxygen dissociation rate, similar to myoglobin. Like myoglobin and hemoglobin, leghemoglobin has a high affinity for carbon monoxide.
In the primary structure of Leghemoglobin A in soybeans, a valine(F7) is found in place where a serine(F7) is in Myoglobin. Without a hydrogen bond fixing the orientation of the proximal histidine side chain the imidazole ring can occupy a staggered conformation between pyrrole nitrogen atoms and can readily move upward to the heme plane. This greatly increases the reactivity of the iron atom and oxygen affinity. In Leghemoglobin A the distal histidine side chain is also rotated away from the bound ligand by formation of a hydrogen bond with Tyrosine.
Heme groups are the same in all known leghemoglobins, but the amino acid sequence of the globin differs slightly depending on bacterial strain and legume species. Even within one leguminous plant, multiple
Oxygen binding affinities of leghemoglobins are between 11 and 24 times higher than oxygen binding affinities of sperm whale myoglobin. Differences in the affinities are due to differential rates of association between the two types of proteins. One explanation of this phenomenon is that in myoglobin, a bound water molecule is stabilized in a pocket surrounding the heme group. This water group must be displaced in order for oxygen to bind. No such water is bound in the analogous pocket of leghemoglobin, so it is easier for an oxygen molecule to approach the leghemoglobin heme. Leghemoglobin has a slow oxygen dissociation rate, similar to myoglobin. Like myoglobin and hemoglobin, leghemoglobin has a high affinity for carbon monoxide.
In the primary structure of Leghemoglobin A in soybeans, a valine(F7) is found in place where a serine(F7) is in Myoglobin. Without a hydrogen bond fixing the orientation of the proximal histidine side chain the imidazole ring can occupy a staggered conformation between pyrrole nitrogen atoms and can readily move upward to the heme plane. This greatly increases the reactivity of the iron atom and oxygen affinity. In Leghemoglobin A the distal histidine side chain is also rotated away from the bound ligand by formation of a hydrogen bond with Tyrosine.
Heme groups are the same in all known leghemoglobins, but the amino acid sequence of the globin differs slightly depending on bacterial strain and legume species. Even within one leguminous plant, multiple
Impossible Burger’s ‘Secret Sauce’ Highlights Challenges of Food TechUpdates FDA Announces Effective Date for Final Rule Adding Soy Leghemoglobin to List of Color Additives Exempt from Certification
{{Globins Hemoproteins Nitrogen cycle Phytochemicals Hemoglobins
 Leghemoglobin (also leghaemoglobin or legoglobin) is an oxygen-carrying phytoglobin found in the
Leghemoglobin (also leghaemoglobin or legoglobin) is an oxygen-carrying phytoglobin found in the nitrogen-fixing
Nitrogen fixation is a chemical process by which molecular dinitrogen () is converted into ammonia (). It occurs both biologically and abiological nitrogen fixation, abiologically in chemical industry, chemical industries. Biological nitrogen ...
root nodule
Root nodules are found on the roots of plants, primarily legumes, that form a symbiosis with nitrogen-fixing bacteria. Under nitrogen-limiting conditions, capable plants form a symbiotic relationship with a host-specific strain of bacteria known ...
s of leguminous plants. It is produced by these plants in response to the roots being colonized by nitrogen-fixing bacteria, termed rhizobia
Rhizobia are diazotrophic bacteria that fix nitrogen after becoming established inside the root nodules of legumes (Fabaceae). To express genes for nitrogen fixation, rhizobia require a plant host; they cannot independently fix nitrogen. I ...
, as part of the symbiotic
Symbiosis (Ancient Greek : living with, companionship < : together; and ''bíōsis'': living) is any type of a close and long-term biolo ...
interaction between plant and bacterium: roots not colonized by ''Rhizobium
''Rhizobium'' is a genus of Gram-negative soil bacteria that fix nitrogen. ''Rhizobium'' species form an endosymbiotic nitrogen-fixing association with roots of (primarily) legumes and other flowering plants.
The bacteria colonize plant ce ...
'' do not synthesise leghemoglobin. Leghemoglobin has close chemical and structural similarities to hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
, and, like hemoglobin, is red in colour. It was originally thought that the heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
prosthetic group for plant leghemoglobin was provided by the bacterial symbiont within symbiotic root nodules. However, subsequent work shows that the plant host strongly expresses heme biosynthesis genes within nodules, and that activation of those genes correlates with leghemoglobin gene expression in developing nodules.
In plants colonised by ''Rhizobium'', such as alfalfa
Alfalfa () (''Medicago sativa''), also called lucerne, is a perennial plant, perennial flowering plant in the legume family Fabaceae. It is cultivated as an important forage crop in many countries around the world. It is used for grazing, hay, ...
or soybean
The soybean, soy bean, or soya bean (''Glycine max'') is a species of legume native to East Asia, widely grown for its edible bean. Soy is a staple crop, the world's most grown legume, and an important animal feed.
Soy is a key source o ...
s, the presence of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
in the root nodules would reduce the activity of the oxygen-sensitive nitrogenase, which is an enzyme responsible for the fixation of atmospheric nitrogen. Leghemoglobin is shown to buffer the concentration of free oxygen in the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
of infected plant cells to ensure the proper function of root nodules. That being said, nitrogen fixation is an extremely energetically costly process, so aerobic respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cellu ...
, which necessitates high oxygen concentration, is necessary in the cells of the root nodule. Leghemoglobin maintains a free oxygen concentration that is low enough to allow nitrogenase to function, but a high enough total oxygen concentration (free and bound to leghemoglobin) for aerobic respiration.
Leghemoglobin falls into the class of symbiotic globins, which also include the root nodules globins of actinorhizal plants such as '' Casuarina''. The ''Casuarina'' symbiotic globin is intermediate between leghemoglobin and nonsymbiotic phytoglobin-2.
Structure
Leghemoglobins are monomeric proteins with a mass around 16 kDa, and are structurally similar tomyoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle, skeletal Muscle, muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compar ...
.Singh S., Varma A. (2017) Structure, Function, and Estimation of Leghemoglobin. In: Hansen A., Choudhary D., Agrawal P., Varma A. (eds) Rhizobium Biology and Biotechnology. Soil Biology, vol 50. Springer, Cham One leghemoglobin protein consists of a heme bound to an iron, and one polypeptide chain (the globin). Similar to myoglobin and hemoglobin, the iron of heme is found in its ferrous
In chemistry, iron(II) refers to the chemical element, element iron in its +2 oxidation number, oxidation state. The adjective ''ferrous'' or the prefix ''ferro-'' is often used to specify such compounds, as in ''ferrous chloride'' for iron(II ...
state in vivo, and is the moiety that binds oxygen. Despite similarities in the mechanism of oxygen binding between leghemoglobin and animal hemoglobin, and the fact that leghemoglobin and animal hemoglobin evolved from a common ancestor, there is dissimilarity in amino acid sequence between these proteins at about 80% of positions.
 Oxygen binding affinities of leghemoglobins are between 11 and 24 times higher than oxygen binding affinities of sperm whale myoglobin. Differences in the affinities are due to differential rates of association between the two types of proteins. One explanation of this phenomenon is that in myoglobin, a bound water molecule is stabilized in a pocket surrounding the heme group. This water group must be displaced in order for oxygen to bind. No such water is bound in the analogous pocket of leghemoglobin, so it is easier for an oxygen molecule to approach the leghemoglobin heme. Leghemoglobin has a slow oxygen dissociation rate, similar to myoglobin. Like myoglobin and hemoglobin, leghemoglobin has a high affinity for carbon monoxide.
In the primary structure of Leghemoglobin A in soybeans, a valine(F7) is found in place where a serine(F7) is in Myoglobin. Without a hydrogen bond fixing the orientation of the proximal histidine side chain the imidazole ring can occupy a staggered conformation between pyrrole nitrogen atoms and can readily move upward to the heme plane. This greatly increases the reactivity of the iron atom and oxygen affinity. In Leghemoglobin A the distal histidine side chain is also rotated away from the bound ligand by formation of a hydrogen bond with Tyrosine.
Heme groups are the same in all known leghemoglobins, but the amino acid sequence of the globin differs slightly depending on bacterial strain and legume species. Even within one leguminous plant, multiple
Oxygen binding affinities of leghemoglobins are between 11 and 24 times higher than oxygen binding affinities of sperm whale myoglobin. Differences in the affinities are due to differential rates of association between the two types of proteins. One explanation of this phenomenon is that in myoglobin, a bound water molecule is stabilized in a pocket surrounding the heme group. This water group must be displaced in order for oxygen to bind. No such water is bound in the analogous pocket of leghemoglobin, so it is easier for an oxygen molecule to approach the leghemoglobin heme. Leghemoglobin has a slow oxygen dissociation rate, similar to myoglobin. Like myoglobin and hemoglobin, leghemoglobin has a high affinity for carbon monoxide.
In the primary structure of Leghemoglobin A in soybeans, a valine(F7) is found in place where a serine(F7) is in Myoglobin. Without a hydrogen bond fixing the orientation of the proximal histidine side chain the imidazole ring can occupy a staggered conformation between pyrrole nitrogen atoms and can readily move upward to the heme plane. This greatly increases the reactivity of the iron atom and oxygen affinity. In Leghemoglobin A the distal histidine side chain is also rotated away from the bound ligand by formation of a hydrogen bond with Tyrosine.
Heme groups are the same in all known leghemoglobins, but the amino acid sequence of the globin differs slightly depending on bacterial strain and legume species. Even within one leguminous plant, multiple isoforms
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have uniqu ...
of leghemoglobins can exist. These often differ in oxygen affinity, and help meet the needs of a cell in a particular environment within the nodule.
Debate on principal function
Results of a 1995 study suggested that the low free oxygen concentration in root nodule cells is actually due to the low oxygen permeability of root nodule cells. It follows that the main purpose of leghemoglobin is to scavenge the limited free oxygen in the cell and deliver it tomitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
for respiration. But, scientists of a later 2005 article suggest that leghemoglobin is responsible both for buffering oxygen concentration, and for delivery of oxygen to mitochondria. Their leghemoglobin knockout
A knockout (abbreviated to KO or K.O.) is a fight-ending, winning criterion in several full-contact combat sports, such as boxing, kickboxing, Muay Thai, mixed martial arts, karate, some forms of taekwondo and other sports involving striking, ...
studies showed that leghemoglobin actually does significantly decrease the free oxygen concentration in root nodule cells, and that nitrogenase expression was eliminated in leghemoglobin knockout mutants, assumably due to the degradation of nitrogenase with high free oxygen concentration. Their study also showed a higher ATP/ ADP ratio in wild-type root nodule cells with active leghemoglobin, suggesting that leghemoglobin also assists with delivery of oxygen for respiration.
Plants contain both symbiotic and nonsymbiotic hemoglobins. Symbiotic hemoglobins are thought to be important for symbiotic nitrogen fixation (SNF). In legume, SNF takes place in specialized organs called nodules which contain bacteroids, or nitrogen fixing rhizobia. The induction of nodule-specific plant genes, which include those that encode for symbiotic leghemoglobins (Lb), accompany nodule development. Leghemoglobins accumulate to millimolar concentrations in the cytoplasm of infected plant cells prior to nitrogen fixation to buffer free oxygen in the nanomolar range, which can avoid inactivation of oxygen-labile nitrogenase while keeping a high enough oxygen flux for respiration in the cell. The leghemoglobins are required for SNF but are not required for plant growth and development in the presence of an external source of fixed nitrogen. Leghemoglobins make the essential contribution of establishing low free-oxygen concentrations while keep a high energy status in cells. These are the conditions necessary for effective SNF.
Other plant hemoglobins
Globins have since been identified as a protein common to many plant taxa, not restricted to symbiotic ones. In light of this discovery, it has been proposed that the term phytoglobins be used for referring to plant globins in general. Phytoglobins can be divided into two clades. The 3/3-fold type contains Classes I and II of angiosperm phytoglobins, and is the one common to all eukaryotes ( HGT of a bacterial flavohemoglobin). The leghemoglobin ''sensu stricto'' is a class II phytoglobin. The 2/2-fold "TrHb2" type contains class III in angiosperm nomenclature, and appears to be acquired from Chloroflexota (formerly Chloroflexi) by the ancestor of land plants.Commercial use
Impossible Foods asked the American FDA for their approval to use recombinant soy leghemoglobin made by ''Pichia pastoris
''Komagataella'' is a methylotrophic yeast within the order Saccharomycetales. It was found in the 1960s as ''Pichia pastoris'', with its feature of using methanol as a source of carbon and energy. In 1995, ''P. pastoris'' was reassigned into t ...
'' in meat alternatives as an analog of meat-derived hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
. Approval from the FDA came in July 2019, was challenged, and later upheld, on May 3, 2021, by a San Francisco federal appeals court. It is currently being used in their products to mimic the color, taste, and texture of meat.
See also
*Hemocyanin
Hemocyanins (also spelled haemocyanins and abbreviated Hc) are proteins that transport oxygen throughout the bodies of some invertebrate animals. These metalloproteins contain two copper atoms that reversibly bind a single oxygen molecule (O2 ...
* Hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
* Myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle, skeletal Muscle, muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compar ...
* Phytoglobin
References
Notes
Further reading
* *Impossible Burger’s ‘Secret Sauce’ Highlights Challenges of Food Tech
{{Globins Hemoproteins Nitrogen cycle Phytochemicals Hemoglobins