lateral flow test on:
[Wikipedia]
[Google]
[Amazon]
 A lateral flow test (LFT), is an
A lateral flow test (LFT), is an
 Sandwich assays are generally used for larger analytes because they tend to have multiple binding sites. As the sample migrates through the assay it first encounters a conjugate, which is an antibody specific to the target analyte labelled with a visual tag, usually colloidal gold. The antibodies bind to the target analyte within the sample and migrate together until they reach the test line. The test line also contains immobilized antibodies specific to the target analyte, which bind to the migrated analyte bound conjugate molecules. The test line then presents a visual change due to the concentrated visual tag, hence confirming the presence of the target molecules. The majority of sandwich assays also have a control line which will appear whether or not the target analyte is present to ensure proper function of the lateral flow pad.
The rapid, low-cost sandwich-based assay is commonly used for home pregnancy tests which detect
Sandwich assays are generally used for larger analytes because they tend to have multiple binding sites. As the sample migrates through the assay it first encounters a conjugate, which is an antibody specific to the target analyte labelled with a visual tag, usually colloidal gold. The antibodies bind to the target analyte within the sample and migrate together until they reach the test line. The test line also contains immobilized antibodies specific to the target analyte, which bind to the migrated analyte bound conjugate molecules. The test line then presents a visual change due to the concentrated visual tag, hence confirming the presence of the target molecules. The majority of sandwich assays also have a control line which will appear whether or not the target analyte is present to ensure proper function of the lateral flow pad.
The rapid, low-cost sandwich-based assay is commonly used for home pregnancy tests which detect
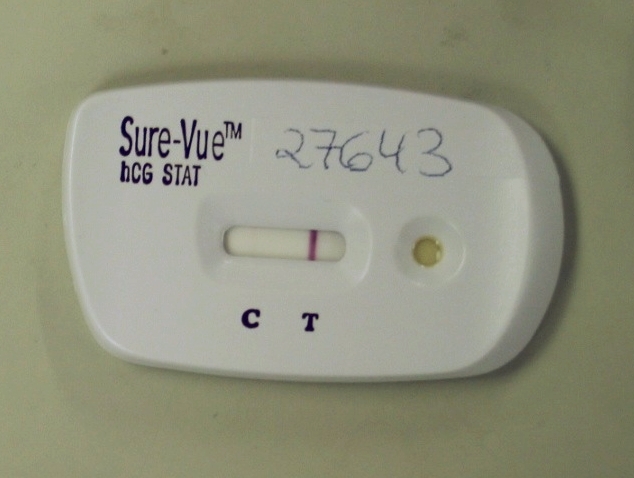 Most tests will incorporate a second line which contains a further antibody (one which is not specific to the analyte) that binds some of the remaining colored particles which did not bind to the test line. This confirms that fluid has passed successfully from the sample-application pad, past the test line. By giving confirmation that the sample has had a chance to interact with the test line, this increases confidence that a visibly-unchanged test line can be interpreted as a negative result (or that a ''changed'' test line can be interpreted as a ''negative'' result in a competitive assay).
Most tests will incorporate a second line which contains a further antibody (one which is not specific to the analyte) that binds some of the remaining colored particles which did not bind to the test line. This confirms that fluid has passed successfully from the sample-application pad, past the test line. By giving confirmation that the sample has had a chance to interact with the test line, this increases confidence that a visibly-unchanged test line can be interpreted as a negative result (or that a ''changed'' test line can be interpreted as a ''negative'' result in a competitive assay).
 A lateral flow test (LFT), is an
A lateral flow test (LFT), is an assay
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity ...
also known as a lateral flow immunochromatographic test (ICT), or rapid test. It is a simple device intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment. LFTs are widely used in medical diagnostics in the home, at the point of care, and in the laboratory. For instance, the home pregnancy test
A pregnancy test is used to determine whether a person is Pregnancy, pregnant or not. The two primary methods are testing for the pregnancy hormone (human chorionic gonadotropin (hCG)) in blood or urine using a pregnancy test kit, and scanning ...
is an LFT that detects a specific hormone. These tests are simple and economical and generally show results in around five to thirty minutes. Many lab-based applications increase the sensitivity of simple LFTs by employing additional dedicated equipment. Because the target substance is often a biological antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
...
, many lateral flow tests are rapid antigen test
A rapid antigen test (RAT), sometimes called a rapid antigen detection test (RADT), antigen rapid test (ART), or wikt:Appendix:Glossary#loosely, loosely just a rapid test, is a rapid diagnostic test suitable for point-of-care testing that directl ...
s (RAT or ART).
LFTs operate on the same principles of affinity chromatography
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the ...
as the enzyme-linked immunosorbent assays (ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of ...
). In essence, these tests run the liquid sample along the surface of a pad with reactive molecules that show a visual positive or negative result. The pads are based on a series of capillary beds, such as pieces of porous paper, microstructure
Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material (such as metals, polymer ...
d polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
, or sintered
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, pla ...
polymer. Each of these pads has the capacity to transport fluid (e.g., urine, blood, saliva) spontaneously.
The sample pad acts as a sponge and holds an excess of sample fluid. Once soaked, the fluid flows to the second conjugate pad in which the manufacturer has stored freeze dried bio-active particles called conjugates (see below) in a salt–sugar matrix. The conjugate pad contains all the reagents required for an optimized chemical reaction between the target molecule (e.g., an antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
...
) and its chemical partner (e.g., antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, includin ...
) that has been immobilized on the particle's surface. This marks target particles as they pass through the pad and continue across to the test and control lines. The test line shows a signal, often a color as in pregnancy tests. The control line contains affinity ligands which show whether the sample has flowed through and the bio-molecules in the conjugate pad are active. After passing these reaction zones, the fluid enters the final porous material, the wick, that simply acts as a waste container.
LFTs can operate as either competitive
Competition is a rivalry where two or more parties strive for a common goal which cannot be shared: where one's gain is the other's loss (an example of which is a zero-sum game). Competition can arise between entities such as organisms, indi ...
or sandwich assays.
History
LFTs derive frompaper chromatography
Paper chromatography is an analytical method used to separate colored chemicals or substances. It can also be used for colorless chemicals that can be located by a stain or other visualisation method after separation. It is now primarily used as ...
, which was developed in 1943 by Martin Martin may refer to:
Places Antarctica
* Martin Peninsula, Marie Byrd Land
* Port Martin, Adelie Land
* Point Martin, South Orkney Islands
Europe
* Martin, Croatia, a village
* Martin, Slovakia, a city
* Martín del Río, Aragón, Spain
* M ...
and Synge, and elaborated in 1944 by Consden, Gordon and Martin. There was an explosion of activity in this field after 1945. The ELISA technology was developed in 1971. A set of LFT patents, including the litigated US 6,485,982 described below, were filed by Armkel LLC starting in 1988.
The first commercially available lateral flow device was Unipath's Clearblue One Step in 1988. This product combined Paired Monoclonal Antibody technology (patented in 1980 by Unipath's Prof. Philip Porter and colleagues including Paul Davis and Keith May) and the original Clearblue product launched in June 1985.
Synopsis
Colored particles
In principle, any colored particle can be used, butlatex
Latex is an emulsion (stable dispersion) of polymer microparticles in water. Latices are found in nature, but synthetic latices are common as well.
In nature, latex is found as a wikt:milky, milky fluid, which is present in 10% of all floweri ...
(blue color) or nanometer-sized particles of gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
(red color) are most commonly used. The gold particles are red in color due to localized surface plasmon resonance
Surface plasmon resonance (SPR) is a phenomenon that occurs where electrons in a thin metal sheet become excited by light that is directed to the sheet with a particular angle of incidence (optics), angle of incidence, and then travel parallel to ...
. Fluorescent
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with color ...
or magnetic
Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, m ...
labelled particles can also be used, but these require the use of an electronic reader to assess the test result.
Sandwich assays
human chorionic gonadotropin
Human chorionic gonadotropin (hCG) is a hormone for the maternal recognition of pregnancy produced by trophoblast cells that are surrounding a growing embryo (syncytiotrophoblast initially), which eventually forms the placenta after implantat ...
, hCG, in the urine
Urine is a liquid by-product of metabolism in humans and many other animals. In placental mammals, urine flows from the Kidney (vertebrates), kidneys through the ureters to the urinary bladder and exits the urethra through the penile meatus (mal ...
of pregnant women.
Competitive assays
Competitive assays are generally used for smaller analytes since smaller analytes have fewer binding sites. The sample first encounters antibodies to the target analyte labelled with a visual tag (colored particles). The test line contains the target analyte fixed to the surface. When the target analyte is absent from the sample, unbound antibody will bind to these fixed analyte molecules, meaning that a visual marker will show. Conversely, when the target analyte is present in the sample, it binds to the antibodies to prevent them binding to the fixed analyte in the test line, and thus no visual marker shows. This differs from sandwich assays in that no band means the analyte is present.Quantitative tests
Most LFTs are intended to operate on a purely qualitative basis. However, it is possible to measure the intensity of the test line to determine the quantity of analyte in the sample. Handheld diagnostic devices known as lateral flow readers are used by several companies to provide a fully quantitative assay result. By utilizing unique wavelengths of light for illumination in conjunction with eitherCMOS
Complementary metal–oxide–semiconductor (CMOS, pronounced "sea-moss
", , ) is a type of MOSFET, metal–oxide–semiconductor field-effect transistor (MOSFET) semiconductor device fabrication, fabrication process that uses complementary an ...
or CCD
CCD may refer to:
Science and technology
* Charge-coupled device, an electronic light sensor used in various devices including digital cameras
* .ccd, the filename extension for CloneCD's CD image file
* Carbonate compensation depth, a property ...
detection technology, a signal rich image can be produced of the actual test lines. Using image processing algorithms specifically designed for a particular test type and medium, line intensities can then be correlated with analyte concentrations. One such handheld lateral flow device platform is made by Detekt Biomedical L.L.C. Alternative non-optical techniques are also able to report quantitative assays results. One such example is a magnetic immunoassay
Magnetic immunoassay (MIA) is a type of diagnostic immunoassay using magnetic beads as labels in lieu of conventional enzymes (ELISA), radioisotopes ( RIA) or fluorescent moieties ( fluorescent immunoassays) to detect a specified analyte. MIA invol ...
(MIA) in the LFT form also allows for getting a quantified result. Reducing variations in the capillary pumping of the sample fluid is another approach to move from qualitative to quantitative results. Recent work has, for example, demonstrated capillary pumping with a constant flow rate independent from the liquid viscosity and surface energy
In surface science, surface energy (also interfacial free energy or surface free energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energe ...
.
Control line
Blood plasma extraction
Because the intense red color ofhemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
interferes with the readout of colorimetric
Colorimetry is "the science and technology used to quantify and describe physically the human color perception".
It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color p ...
or optical detection-based diagnostic tests, blood plasma
Blood plasma is a light Amber (color), amber-colored liquid component of blood in which blood cells are absent, but which contains Blood protein, proteins and other constituents of whole blood in Suspension (chemistry), suspension. It makes up ...
separation is a common first step to increase diagnostic test accuracy. Plasma can be extracted from whole blood via integrated filters or via agglutination.
Speed and simplicity
Time to obtain the test result is a key driver for these products. Tests results can be available in as little as a few minutes. Generally there is a trade off between time and sensitivity: more sensitive tests may take longer to develop. The other key advantage of this format of test compared to other immunoassays is the simplicity of the test, by typically requiring little or no sample or reagent preparation.Patents
This is a highly competitive area and a number of people claim patents in the field, most notably Alere (formerly Inverness Medical Innovations, now owned by Abbott) who own patents originally filed by Unipath. The US 6,485,982 patent, that has been litigated, expired in 2019. A number of other companies also hold patents in this arena. A group of competitors are challenging the validity of the patents. The original patent is apparently from 1988.Applications
Lateral flow assays have a wide array of applications and can test a variety of samples including urine, blood, saliva, sweat, serum, and other fluids. They are currently used by clinical laboratories, hospitals, physicians and veterinary clinics, food analysis labs and environmental testing facilities. Immediacy in obtaining results is normally the key factor in choosing this technique, although simplicity and lack of a need for formal equipment are also important factors. These features allow ICTs to be used a at-home test or in pharmacies. Because of their exceptional quality, rapid test are also used routinely in well-equippped laboratories when the demand for test is low. The broad applications of rapid test can be realized because of their simplicity accompanied by high quality analytical production. The sensitivity and specificity of these techniques tend to be comparable to those of other more complex methods, and on occasion significantly better. Other uses for lateral flow assays are food and environmental safety and veterinary medicine for chemicals such as diseases and toxins. LFTs are also commonly used for disease identification such as ebola, but the most common LFT are the home pregnancy and SARS-CoV-2 tests.COVID-19 testing
Lateral flow assays have played a critical role inCOVID-19
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by the coronavirus SARS-CoV-2. In January 2020, the disease spread worldwide, resulting in the COVID-19 pandemic.
The symptoms of COVID‑19 can vary but often include fever ...
testing as they have the benefit of delivering a result in 15–30 minutes. The systematic evaluation of lateral flow assays during the COVID-19 pandemic was initiated at Oxford University
The University of Oxford is a collegiate research university in Oxford, England. There is evidence of teaching as early as 1096, making it the oldest university in the English-speaking world and the second-oldest continuously operating u ...
as part of a UK collaboration with Public Health England
Public Health England (PHE) was an executive agency of the Department of Health and Social Care in England which began operating on 1 April 2013 to protect and improve health and wellbeing and reduce health inequalities. Its formation came as a ...
. A study that started in June 2020 in the United Kingdom, FALCON-C19, confirmed the sensitivity of some lateral flow devices (LFDs) in this setting. Four out of 64 LFDs tested had desirable performance characteristics according to these early tests; the Innova SARS-CoV-2 Antigen Rapid Qualitative Test performed moderately in viral antigen detection/sensitivity with excellent specificity, although kit failure rates and the impact of training were potential issues. The Innova test's specificity is more widely publicised, but sensitivity in phase 4 trials was 50.1%. This describes a device for which one out of every two patients infected with COVID-19 and tested in real-world conditions would receive a false-negative result. After closure of schools in January 2021, biweekly LFTs were introduced in England for teachers, pupils, and households of pupils when schools re-opened on March 8, 2021 for asymptomatic testing. Biweekly LFT were made universally available to everyone in England on April 9, 2021. LFTs have been used for mass testing for COVID-19 globally and complement other public health measures for COVID-19.
Some scientists outside government expressed serious misgivings in late 2020 about the use of Innova LFDs for screening for Covid. According to Jon Deeks, a professor of biostatistics at the University of Birmingham
The University of Birmingham (informally Birmingham University) is a Public university, public research university in Birmingham, England. It received its royal charter in 1900 as a successor to Queen's College, Birmingham (founded in 1825 as ...
, England, the Innova test is "entirely unsuitable" for community testing: "as the test may miss up to half of cases, a negative test result indicates a reduced risk of Covid, but does not exclude Covid".
Sensitivity of tests used in 2022 was around 70%.
See also
* : LFT test for ovulationReferences
Further reading
* * Porex Clinical Sciences (manufacturer) {{Cite web , url=http://www.porex.com/products/clinical-sciences/in-vitro-diagnostics/ , title=Sample Collection & Transport | Sample Preparation | Sample Analysis Medical terminology Molecular biology Biotechnology Molecular biology techniques Chromatography Immunologic tests