Lasso Peptide on:
[Wikipedia]
[Google]
[Amazon]
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of


 Cyanobactins are diverse metabolites from
Cyanobactins are diverse metabolites from
 Lanthipeptides are one of the most well-studied families of RiPPs. The family is characterized by the presence of
Lanthipeptides are one of the most well-studied families of RiPPs. The family is characterized by the presence of
 Linear azole(in)e-containing peptides (LAPs) contain
Linear azole(in)e-containing peptides (LAPs) contain
 Most of the characterized thiopeptides have been isolated from Actinobacteria. General structural features of thiopeptide
Most of the characterized thiopeptides have been isolated from Actinobacteria. General structural features of thiopeptide
 All RiPPs are synthesized first at the ribosome as a precursor peptide. This peptide consists of a core peptide segment which is typically preceded (and occasionally followed) by a leader peptide segment and is typically ~20-110 residue (chemistry), residues long. The leader peptide is usually important for enabling enzymatic processing of the precursor peptide via aiding in recognition of the core peptide by biosynthetic enzymes and for cellular export. Some RiPPs also contain a recognition sequence C-terminal to the core peptide; these are involved in excision and cyclization. Additionally, eukaryotic RiPPs may contain a signal segment of the precursor peptide which helps direct the peptide to cellular compartments.
During RiPP biosynthesis, the unmodified precursor peptide (containing an unmodified core peptide, UCP) is recognized and chemically modified sequentially by biosynthetic enzymes (PRPS). Examples of modifications include dehydration (i.e. lantibiotics, lanthipeptides, thiopeptides), cyclodehydration (i.e. thiopeptides), prenylation (i.e. cyanobactins), and cyclization (i.e. lasso peptides), among others. The resulting modified precursor peptide (containing a modified core peptide, MCP) then undergoes proteolysis, wherein the non-core regions of the precursor peptide are removed. This results in the mature RiPP.
All RiPPs are synthesized first at the ribosome as a precursor peptide. This peptide consists of a core peptide segment which is typically preceded (and occasionally followed) by a leader peptide segment and is typically ~20-110 residue (chemistry), residues long. The leader peptide is usually important for enabling enzymatic processing of the precursor peptide via aiding in recognition of the core peptide by biosynthetic enzymes and for cellular export. Some RiPPs also contain a recognition sequence C-terminal to the core peptide; these are involved in excision and cyclization. Additionally, eukaryotic RiPPs may contain a signal segment of the precursor peptide which helps direct the peptide to cellular compartments.
During RiPP biosynthesis, the unmodified precursor peptide (containing an unmodified core peptide, UCP) is recognized and chemically modified sequentially by biosynthetic enzymes (PRPS). Examples of modifications include dehydration (i.e. lantibiotics, lanthipeptides, thiopeptides), cyclodehydration (i.e. thiopeptides), prenylation (i.e. cyanobactins), and cyclization (i.e. lasso peptides), among others. The resulting modified precursor peptide (containing a modified core peptide, MCP) then undergoes proteolysis, wherein the non-core regions of the precursor peptide are removed. This results in the mature RiPP.
 The hallmark of linear azol(in)e-containing peptide (LAP) biosynthesis is the formation of azole, azol(in)e heterocyclic compound, heterocycles from the nucleophile, nucleophilic amino acids
The hallmark of linear azol(in)e-containing peptide (LAP) biosynthesis is the formation of azole, azol(in)e heterocyclic compound, heterocycles from the nucleophile, nucleophilic amino acids
 Lasso peptide biosynthesis requires at least three genes, referred to as the A, B, and C proteins. The A gene encodes the precursor peptide, which is modified by the B and C proteins into the mature natural product. The B protein is an adenosine triphosphate-dependent cysteine protease that cleaves the leader region from the precursor peptide. The C protein displays Homologous series, homology to asparagine synthetase and is thought to activate the carboxylic acid side chain of a
Lasso peptide biosynthesis requires at least three genes, referred to as the A, B, and C proteins. The A gene encodes the precursor peptide, which is modified by the B and C proteins into the mature natural product. The B protein is an adenosine triphosphate-dependent cysteine protease that cleaves the leader region from the precursor peptide. The C protein displays Homologous series, homology to asparagine synthetase and is thought to activate the carboxylic acid side chain of a
ribosomal
Ribosomes () are macromolecular machines, found within all cells, that perform biological protein synthesis (messenger RNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA molecules to fo ...
origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms
An organism is any living thing that functions as an individual. Such a definition raises more problems than it solves, not least because the concept of an individual is also difficult. Many criteria, few of them widely accepted, have been pr ...
, including prokaryotes
A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' ...
, eukaryotes
The eukaryotes ( ) constitute the domain of Eukaryota or Eukarya, organisms whose cells have a membrane-bound nucleus. All animals, plants, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of ...
, and archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
, and they possess a wide range of biological functions.
As a consequence of the falling cost of genome sequencing
Whole genome sequencing (WGS), also known as full genome sequencing or just genome sequencing, is the process of determining the entirety of the DNA sequence of an organism's genome at a single time. This entails sequencing all of an organism's ...
and the accompanying rise in available genomic data, scientific interest in RiPPs has increased in the last few decades. Because the chemical structures of RiPPs are more closely predictable from genomic data than are other natural products (e.g. alkaloids
Alkaloids are a broad class of naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large variety of organisms i ...
, terpenoids
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes" ...
), their presence in sequenced organisms can, in theory, be identified rapidly. This makes RiPPs an attractive target of modern natural product discovery efforts.
Definition
RiPPs consist of anypeptides
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Dalton (unit), Da or more are called proteins. Chains of fewer t ...
(i.e. molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
below 10 kDa) that are ribosomally-produced and undergo some degree of enzymatic post-translational modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translation (biolog ...
. This combination of peptide translation and modification is referred to as "post-ribosomal peptide synthesis" (PRPS) in analogy with nonribosomal peptide synthesis (NRPS).
Historically, the current sub-classes of RiPPs were studied individually, and common practices in nomenclature
Nomenclature (, ) is a system of names or terms, or the rules for forming these terms in a particular field of arts or sciences. (The theoretical field studying nomenclature is sometimes referred to as ''onymology'' or ''taxonymy'' ). The principl ...
varied accordingly in the literature. More recently, with the advent of broad genome sequencing, it has been realized that these natural products share a common biosynthetic
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme- catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve ...
origin. In 2013, a set of uniform nomenclature
Nomenclature (, ) is a system of names or terms, or the rules for forming these terms in a particular field of arts or sciences. (The theoretical field studying nomenclature is sometimes referred to as ''onymology'' or ''taxonymy'' ). The principl ...
guidelines were agreed upon and published by a large group of researchers in the field. Prior to this report, RiPPs were referred to by a variety of designations, including ''post-ribosomal peptides'', ''ribosomal natural products'', and ''ribosomal peptides''.
The acronym
An acronym is a type of abbreviation consisting of a phrase whose only pronounced elements are the initial letters or initial sounds of words inside that phrase. Acronyms are often spelled with the initial Letter (alphabet), letter of each wor ...
"RiPP" stands for "ribosomally synthesized and post-translationally modified peptide".
Prevalence and applications
RiPPs constitute one of the major superfamilies ofnatural products
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
, like alkaloids
Alkaloids are a broad class of naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large variety of organisms i ...
, terpenoids
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes" ...
, and nonribosomal peptide
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacterium, bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be ma ...
s, although they tend to be large, with molecular weights
The molecular mass () is the mass of a given molecule, often expressed in units of daltons (Da). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The derived quanti ...
commonly in excess of 1000 Da. The advent of next-generation sequencing
Massive parallel sequencing or massively parallel sequencing is any of several high-throughput approaches to DNA sequencing using the concept of massively parallel processing; it is also called next-generation sequencing (NGS) or second-generation ...
methods has made genome
A genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding genes, other functional regions of the genome such as ...
mining
Mining is the Resource extraction, extraction of valuable geological materials and minerals from the surface of the Earth. Mining is required to obtain most materials that cannot be grown through agriculture, agricultural processes, or feasib ...
of RiPPs a common strategy. In part due to their increased discovery and hypothesized ease of engineering
Engineering is the practice of using natural science, mathematics, and the engineering design process to Problem solving#Engineering, solve problems within technology, increase efficiency and productivity, and improve Systems engineering, s ...
, the use of RiPPs as drugs
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via inhalation, injection, smoking, ingestio ...
is increasing. Although they are ribosomal
Ribosomes () are macromolecular machines, found within all cells, that perform biological protein synthesis (messenger RNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA molecules to fo ...
peptides
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Dalton (unit), Da or more are called proteins. Chains of fewer t ...
in origin, RiPPs are typically categorized as small molecules
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; t ...
rather than biologics
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, th ...
due to their chemical properties, such as moderate molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
and relatively high hydrophobicity
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly intermolecular force, repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to b ...
.
The uses and biological activities of RiPPs are diverse.
RiPPs in commercial use include nisin
Nisin is a polycyclic antibacterial peptide produced by the bacterium ''Lactococcus lactis'' that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), dideh ...
, a food preservative
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or ...
, thiostrepton
Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as '' Streptomyces azureus'' and '' Streptomyces laurentii''. Thiostrepton is a natural product of the ribo ...
, a veterinary
Veterinary medicine is the branch of medicine that deals with the prevention, management, diagnosis, and treatment of disease, disorder, and injury in non-human animals. The scope of veterinary medicine is wide, covering all animal species, both ...
topical antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention o ...
, and nosiheptide
Nosiheptide is a thiopeptide antibiotic produced by the bacterium ''Streptomyces actuosus''.
Chemical classification
Nosiheptide is classified, along with several others, as an e series thiopeptide characterized by a nitrogen containing, 6-memb ...
and duramycin, which are animal
Animals are multicellular, eukaryotic organisms in the Biology, biological Kingdom (biology), kingdom Animalia (). With few exceptions, animals heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, ...
feed additives A feed additive is an additive of extra nutrient or drug for livestock. Such additives include vitamins, amino acids, fatty acids, minerals, pharmaceutical, fungus, fungal products and steroidal compounds. The additives might impact feed presentatio ...
. Phalloidin
Phalloidin belongs to a class of toxins called phallotoxins, which are found in the death cap mushroom ''( Amanita phalloides)''. It is a rigid bicyclic heptapeptide that is lethal after a few days when injected into the bloodstream. The major s ...
functionalized with a fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with se ...
is used in microscopy
Microscopy is the technical field of using microscopes to view subjects too small to be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of microscopy: optical mic ...
as a stain
A stain is a discoloration that can be clearly distinguished from the surface, material, or medium it is found upon. They are caused by the chemical or physical interaction of two dissimilar materials. Accidental staining may make materials app ...
due to its high affinity for actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ...
. Anantin is a RiPP used in cell biology
Cell biology (also cellular biology or cytology) is a branch of biology that studies the structure, function, and behavior of cells. All living organisms are made of cells. A cell is the basic unit of life that is responsible for the living an ...
as an atrial natriuretic peptide receptor
An atrial natriuretic peptide receptor is a receptor for atrial natriuretic peptide.
Mechanism
NPRA and NPRB are linked to guanylyl cyclases, while NPRC is G-protein-linked and is a "clearance receptor" that acts to internalise and destroy the ...
inhibitor
Inhibitor or inhibition may refer to:
Biology
* Enzyme inhibitor, a substance that binds to an enzyme and decreases the enzyme's activity
* Reuptake inhibitor, a substance that increases neurotransmission by blocking the reuptake of a neurotransmi ...
.
In 2012-2013, a derivatized RiPP in clinical trials
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel v ...
was LFF571. Phase II clinical trials
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases ...
of LFF571, a derivative of the thiopeptide GE2270-A, for the treatment of ''Clostridioides difficile
''Clostridioides difficile'' ( syn. ''Clostridium difficile'') is a bacterium known for causing serious diarrheal infections, and may also cause colon cancer. It is known also as ''C. difficile'', or ''C. diff'' (), and is a Gram-positive spec ...
'' infections, with comparable safety and efficacy to vancomycin
Vancomycin is a glycopeptide antibiotic medication used to treat certain bacterial infections. It is administered intravenously ( injection into a vein) to treat complicated skin infections, bloodstream infections, endocarditis, bone an ...
, was terminated early as the results were unfavorable. Also recently in clinical trials was the NVB302 (a derivative of the lantibiotic
Lantibiotics are a class of polycyclic peptide antibiotics that contain the characteristic thioether amino acids lanthionine or methyllanthionine, as well as the unsaturated amino acids dehydroalanine, and 2-aminoisobutyric acid. They belong ...
actagardine) which is used for the treatment of ''Clostridioides difficile'' infection. Duramycin has completed phase II clinical trials for the treatment of cystic fibrosis
Cystic fibrosis (CF) is a genetic disorder inherited in an autosomal recessive manner that impairs the normal clearance of Sputum, mucus from the lungs, which facilitates the colonization and infection of the lungs by bacteria, notably ''Staphy ...
.
Other bioactive RiPPs include the antibiotics cyclothiazomycin
The cyclothiazomycins are a group of natural products, classified as thiopeptides, which are produced by various ''Streptomyces'' species of bacteria.
These compounds are ribosomally synthesized and post-translationally modified peptides (RiPPs) ...
and bottromycin
Bottromycin is a macrocyclic peptide with antibiotic activity. It was first discovered in 1957 as a natural product isolated from ''Streptomyces bottropensis''. It has been shown to inhibit methicillin-resistant ''Staphylococcus aureus'' (MRSA) and ...
, the ultra-narrow spectrum antibiotic plantazolicin
Plantazolicin (PZN) is a natural antibiotic produced by the gram-positive soil bacterium ''Bacillus velezensis'' FZB42 (previously '' Bacillus amyloliquefaciens'' FZB42). PZN has specifically been identified as a selective bactericidal agent ac ...
, and the cytotoxin
Cytotoxicity is the quality of being toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium ...
patellamide A. Streptolysin
Streptolysins are two homogenous exotoxins from ''Streptococcus pyogenes''. Types include streptolysin O (SLO; ''slo''), which is oxygen-labile, and streptolysin S (SLS; ''sagA''), which is oxygen-stable.
SLO is part of the thiol-activated c ...
S, the toxic virulence factor
Virulence factors (preferably known as pathogenicity factors or effectors in botany) are cellular structures, molecules and regulatory systems that enable microbial pathogens (bacteria, viruses, fungi, and protozoa) to achieve the following:
* c ...
of ''Streptococcus pyogenes
''Streptococcus pyogenes'' is a species of Gram-positive, aerotolerant bacteria in the genus '' Streptococcus''. These bacteria are extracellular, and made up of non-motile and non-sporing cocci (round cells) that tend to link in chains. They ...
'', is also a RiPP. Additionally, human thyroid hormone
File:Thyroid_system.svg, upright=1.5, The thyroid system of the thyroid hormones triiodothyronine, T3 and T4
rect 376 268 820 433 Thyroid-stimulating hormone
rect 411 200 849 266 Thyrotropin-releasing hormone
rect 297 168 502 200 Hypothalamus
r ...
itself is a RiPP due to its biosynthetic origin as thyroglobulin
Thyroglobulin (Tg) is a 660 kDa, dimeric glycoprotein produced by the follicular cells of the thyroid and used entirely within the thyroid gland. Tg is secreted and accumulated at hundreds of grams per litre in the extracellular compartment ...
.
Classifications
Amatoxins and phallotoxins

Amatoxins
Amatoxins are a subgroup of at least nine related cyclic peptide toxins found in three genera of deadly poisonous mushrooms (''Amanita'', '' Galerina'' and '' Lepiota'') and one species of the genus '' Pholiotina''. Amatoxins are very potent, as li ...
and phallotoxins
The phallotoxins consist of at least seven compounds, all of which are bicyclic heptapeptides (seven amino acids), isolated from the death cap mushroom ''( Amanita phalloides)''. They differ from the closely related amatoxins by being one residue ...
are 8- and 7-membered natural products, respectively, characterized by N-to-C cyclization in addition to a tryptathionine motif derived from the crosslinking of Cys and Trp. The amatoxins and phallotoxins also differ from other RiPPs based on the presence of a C-terminal recognition sequence in addition to the N-terminal leader peptide. α-Amanitin
α-Amanitin (''alpha''-Amanitin) is a cyclic peptide of eight amino acids. It is possibly the most deadly of all the amatoxins, toxins found in several species of the mushroom genus ''Amanita'', one being the death cap (''Amanita phalloides'') as ...
, an amatoxin, has a number of posttranslational modifications in addition to macrocyclization and formation of the tryptathionine bridge: oxidation of the tryptathionine leads to the presence of a sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
, and numerous hydroxylation
In chemistry, hydroxylation refers to the installation of a hydroxyl group () into an organic compound. Hydroxylations generate alcohols and phenols, which are very common functional groups. Hydroxylation confers some degree of water-solubility ...
s decorate the natural product. As an amatoxin, α-amanitin is an inhibitor of RNA polymerase II
RNA polymerase II (RNAP II and Pol II) is a Protein complex, multiprotein complex that Transcription (biology), transcribes DNA into precursors of messenger RNA (mRNA) and most small nuclear RNA (snRNA) and microRNA. It is one of the three RNA pol ...
.
Bottromycins

Bottromycin
Bottromycin is a macrocyclic peptide with antibiotic activity. It was first discovered in 1957 as a natural product isolated from ''Streptomyces bottropensis''. It has been shown to inhibit methicillin-resistant ''Staphylococcus aureus'' (MRSA) and ...
s contain a C-terminal decarboxylated thiazole
Thiazole (), or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the ...
in addition to a macrocyclic amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines includ ...
.
There are currently six known bottromycin compounds, which differ in the extent of side chain methylation, an additional characteristic of the bottromycin class. The total synthesis of bottromycin A2 was required to definitively determine the structure of the first bottromycin.
Thus far, gene clusters predicted to produce bottromycins have been identified in the genus ''Streptomyces
''Streptomyces'', from στρεπτός (''streptós''), meaning "twisted", and μύκης (''múkés''), meaning "fungus", is the largest genus of Actinomycetota, and the type genus of the family Streptomycetaceae. Over 700 species of ''St ...
''. Bottromycins differ from other RiPPs in that there is no N-terminal leader peptide. Rather, the precursor peptide has a C-terminal extension of 35-37 amino acids, hypothesized to act as a recognition sequence for posttranslational machinery.
Cyanobactins
 Cyanobactins are diverse metabolites from
Cyanobactins are diverse metabolites from cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
with N-to-C macrocylization of a 6–20 amino acid chain. Cyanobactins are natural products isolated from cyanobacteria, and close to 30% of all cyanobacterial strains are thought to contain cyanobacterial gene clusters. However, while thus far all cyanobactins are credited to cyanobacteria, there exists the possibility that other organisms could produce similar natural products.
The precursor peptide of the cyanobactin family is traditionally designated the "E" gene, whereas precursor peptides are designated gene "A" in most RiPP gene clusters. "A" is a serine protease involved in cleavage of the leader peptide and subsequent macrocyclization of the peptide natural product, in combination with an additional serine protease homologue, the encoded by gene "G". Members of the cyanobactin family may bear thiazolines/oxazolines, thiazoles/oxazoles, and methylations depending on additional modification enzymes. For example, perhaps the most famous cyanobactin is patellamide A, which contains two thiazoles, a methyloxazoline, and an oxazoline in its final state, a macrocycle derived from 8 amino acids.
Lanthipeptides
 Lanthipeptides are one of the most well-studied families of RiPPs. The family is characterized by the presence of
Lanthipeptides are one of the most well-studied families of RiPPs. The family is characterized by the presence of lanthionine
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element ...
(Lan) and 3-methyllanthionine (MeLan) residues in the final natural product. There are four major classes of lanthipeptides, delineated by the enzymes responsible for installation of Lan and MeLan. The dehydratase and cyclase can be two separate proteins or one multifunctional enzyme. Previously, lanthipeptides were known as "lantipeptides" before a consensus was reached in the field.
Lantibiotics
Lantibiotics are a class of polycyclic peptide antibiotics that contain the characteristic thioether amino acids lanthionine or methyllanthionine, as well as the Saturated and unsaturated compounds, unsaturated amino acids dehydroalanine, and 2-A ...
are lanthipeptides that have known antimicrobial activity. The founding member of the lanthipeptide family, nisin
Nisin is a polycyclic antibacterial peptide produced by the bacterium ''Lactococcus lactis'' that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), dideh ...
, is a lantibiotic that has been used to prevent the growth of food-born pathogens for over 40 years.
Lasso peptides
Lasso peptides are short peptides containing an N-terminal macrolactammacrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
"ring" through which a linear C-terminal "tail" is threaded. Because of this threaded-loop topology
Topology (from the Greek language, Greek words , and ) is the branch of mathematics concerned with the properties of a Mathematical object, geometric object that are preserved under Continuous function, continuous Deformation theory, deformat ...
, these peptides resemble lasso
A lasso or lazo ( or ), also called reata or la reata in Mexico, and in the United States riata or lariat (from Mexican Spanish lasso for roping cattle), is a loop of rope designed as a restraint to be thrown around a target and tightened when ...
s, giving rise to their name. They are a member of a larger class of amino-acid-based lasso structures. Additionally, lasso peptides are formally rotaxanes
A rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle (see graphical representation). The two components of a rotaxane are kinetically trapped since ...
.
The N-terminal "ring" can be from 7 to 9 amino acids long and is formed by an isopeptide bond between the N-terminal amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
of the first amino acid of the peptide and the carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,... ...
side chain of an aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protein ...
or glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that ...
residue. The C-terminal "tail" ranges from 7 to 15 amino acids in length.
The first amino acid of lasso peptides is almost invariably glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
or cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
, with mutations
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mitosi ...
at this site not being tolerated by known enzymes. Thus, bioinformatics
Bioinformatics () is an interdisciplinary field of science that develops methods and Bioinformatics software, software tools for understanding biological data, especially when the data sets are large and complex. Bioinformatics uses biology, ...
-based approaches to lasso peptide discovery have thus used this as a constraint. However, some lasso peptides were recently discovered that also contain serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
or alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group sid ...
as their first residue.
The threading of the lasso tail is trapped either by disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
bonds between ring and tail cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
residues (class I lasso peptides), by steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
due to bulky residues on the tail (class II lasso peptides), or both (class III lasso peptides). The compact structure makes lasso peptides frequently resistant to protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
s or thermal unfolding.
Linear azol(in)e-containing peptides
 Linear azole(in)e-containing peptides (LAPs) contain
Linear azole(in)e-containing peptides (LAPs) contain thiazole
Thiazole (), or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the ...
s and oxazole
Oxazole is the parent compound for a vast class of heterocyclic compound, heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiaz ...
s, or their reduced thiazoline
Thiazolines (; or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivative (chemistry), der ...
and oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the ...
forms. Thiazol(in)es are the result of cyclization of Cys residues in the precursor peptide, while (methyl)oxazol(in)es are formed from Thr and Ser. Azole and azoline formation also modifies the residue in the -1 position, or directly ''C''-terminal to the Cys, Ser, or Thr. A dehydrogenase
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as ...
in the LAP gene cluster
A gene cluster is a group of two or more genes found within an organism's DNA that encode similar peptide, polypeptides or proteins which collectively share a generalized function and are often located within a few thousand base pairs of each othe ...
is required for oxidation of azolines to azoles.
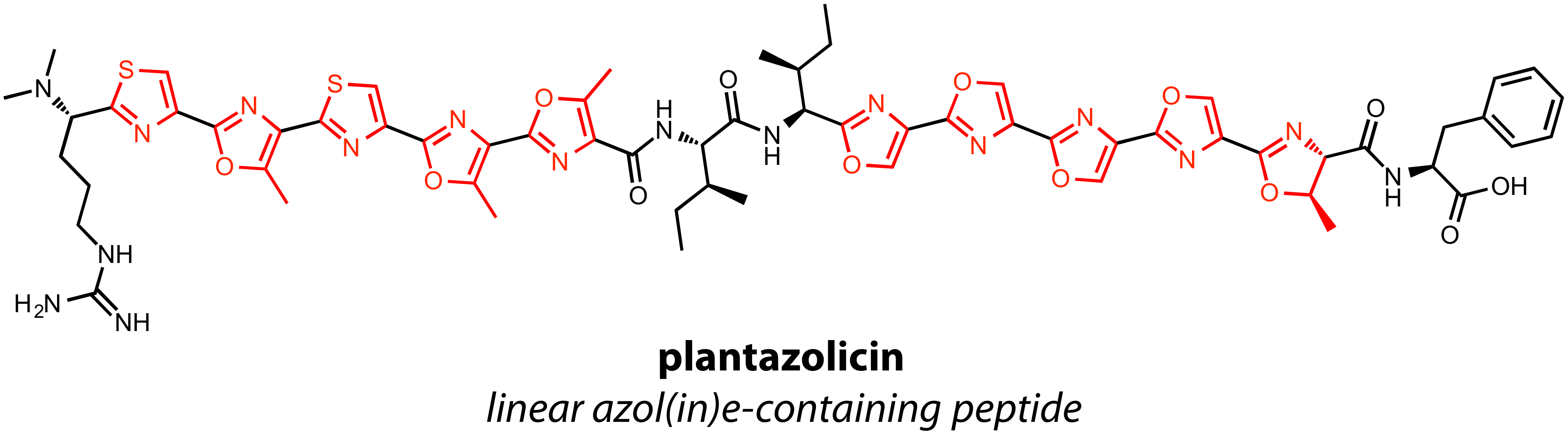
Plantazolicin
Plantazolicin (PZN) is a natural antibiotic produced by the gram-positive soil bacterium ''Bacillus velezensis'' FZB42 (previously '' Bacillus amyloliquefaciens'' FZB42). PZN has specifically been identified as a selective bactericidal agent ac ...
is a LAP with extensive cyclization. Two sets of five heterocycles endow the natural product with structural rigidity and unusually selective antibacterial activity. Streptolysin
Streptolysins are two homogenous exotoxins from ''Streptococcus pyogenes''. Types include streptolysin O (SLO; ''slo''), which is oxygen-labile, and streptolysin S (SLS; ''sagA''), which is oxygen-stable.
SLO is part of the thiol-activated c ...
S (SLS) is perhaps the most well-studied and most famous LAP, in part because the structure is still unknown since the discovery of SLS in 1901. Thus, while the biosynthetic gene cluster suggests SLS is a LAP, structural confirmation is lacking.
Microcins
Microcins are all RiPPs produced byEnterobacteriaceae
Enterobacteriaceae is a large family (biology), family of Gram-negative bacteria. It includes over 30 genera and more than 100 species. Its classification above the level of Family (taxonomy), family is still a subject of debate, but one class ...
with a molecular weight <10 kDa. Many members of other RiPP families, such as microcin E492, microcin B17 (LAP) and microcin J25 (Lasso peptide) are also considered microcins. Instead of being classified based on posttranslational modifications or modifying enzymes, microcins are instead identified by molecular weight, native producer, and antibacterial activity. Microcins are either plasmid- or chromosome-encoded, but specifically have activity against Enerobacteriaceae. Because these organisms are also often producers of microcins, the gene cluster contains not only a precursor peptide and modification enzymes, but also a self-immunity gene to protect the producing strain, and genes encoding export of the natural product.
Microcins have bioactivity against Gram-negative
Gram-negative bacteria are bacteria that, unlike gram-positive bacteria, do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is that their cell envelope consists ...
bacteria but usually display narrow-spectrum activity due to hijacking of specific receptors involved in the transport of essential nutrients.
Thiopeptides
 Most of the characterized thiopeptides have been isolated from Actinobacteria. General structural features of thiopeptide
Most of the characterized thiopeptides have been isolated from Actinobacteria. General structural features of thiopeptide macrocycles
Macrocycles are often described as molecules and ions containing a Ring (chemistry), ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area ...
, are dehydrated amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
and thiazole
Thiazole (), or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the ...
rings formed from dehydrated serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
/threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− ...
and cyclized cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
residues, respectively
The thiopeptide macrocycle is closed with a six-membered nitrogen-bearing ring. Oxidation state and substitution pattern of the nitrogenous ring determines the series of the thiopeptide natural product. While the mechanism of macrocyclization is not known, the nitrogenous ring can exist in thiopeptides as a piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor de ...
, dehydropiperidine, or a fully oxidized pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
. Additionally, some thiopeptides bear a second macrocycle, which bears a quinaldic acid or indolic acid residue derived from tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
. Perhaps the most well-characterized thiopeptide, thiostrepton A, contains a dehydropiperidine ring and a second, quinaldic acid-containing macrocycle. Four residues are dehydrated during posttranslational modification, and the final natural product also bears four thiazoles and one azoline.
Other RiPPs
'' Autoinducing Peptides'' (AIPs) andquorum sensing
In biology, quorum sensing or quorum signaling (QS) is the process of cell-to-cell communication that allows bacteria to detect and respond to cell population density by gene regulation, typically as a means of acclimating to environmental disadv ...
peptides are used as signaling molecules in the process called quorum sensing
In biology, quorum sensing or quorum signaling (QS) is the process of cell-to-cell communication that allows bacteria to detect and respond to cell population density by gene regulation, typically as a means of acclimating to environmental disadv ...
. AIPs are characterized by the presence of a cyclic ester
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated.
Lactones are formed by lactonization, the intramolecular esterification of the corres ...
or thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
, unlike other regulatory peptides that are linear. In pathogens
In biology, a pathogen (, "suffering", "passion" and , "producer of"), in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ.
The term ...
, exported AIPs bind to extracellular receptors that trigger the production of virulence factors
Virulence factors (preferably known as pathogenicity factors or effectors in botany) are cellular structures, molecules and regulatory systems that enable microbial pathogens (bacteria, viruses, fungi, and protozoa) to achieve the following:
* co ...
. In ''Staphylococcus aureus
''Staphylococcus aureus'' is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often posi ...
'', AIPs are biosynthesized from a precursor peptide composed of a C-terminal leader region, the core region, and negatively charged tail region that is, along with the leader peptide, cleaved before AIP export.
''Bacterial Head-to-Tail Cyclized Peptides'' refers exclusively to ribosomally synthesized peptides with 35-70 residues and a peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
between the N- and C-termini, sometimes referred to as bacteriocins
Bacteriocins are proteinaceous or peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s). They are similar to yeast and paramecium killing factors, and are structurally, functionally, and ec ...
, although this term is used more broadly. The distinctive nature of this class is not only the relatively large size of the natural products but also the modifying enzymes responsible for macrocyclization. Other N-to-C cyclized RiPPs, such as the cyanobactins and orbitides, have specialized biosynthetic machinery for macrocylization of much smaller core peptides. Thus far, these bacteriocins have been identified only in Gram-positive bacteria
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
The Gram stain ...
. Enterocin AS-48 was isolated from ''Enterococcus
''Enterococcus'' is a large genus of lactic acid bacteria of the phylum Bacillota. Enterococci are Gram-positive cocci that often occur in pairs ( diplococci) or short chains, and are difficult to distinguish from streptococci on physical ch ...
'' and, like other bacteriocins, is relatively resistant to high temperature, pH changes, and many proteases as a result of macrocyclization. Based on solution structures and sequence alignments, bacteriocins appear to take on similar 3D structures despite little sequence homology, contributing to stability and resistance to degradation.
'' Conopeptides'' and other toxoglossan peptides are the components of the venom
Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved ''venom apparatus'', such as fangs or a sti ...
of predatory marine snails, such as the cone snails or ''Conus
''Conus'' is a genus of venomous and predatory cone snails.Bouchet, P.; Gofas, S. (2015). Conus Linnaeus, 1758. In: MolluscaBase (2015). Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&i ...
''. Venom peptides from cone snails are generally smaller than those found in other animal venoms (10-30 amino acids vs. 30-90 amino acids) and have more disulfide crosslinks. A single species may have 50-200 conopeptides encoded in its genome, recognizable by a well-conserved signal sequence.
''Cyclotide
In biochemistry, cyclotides are small, disulfide-rich peptides isolated from plants. Typically containing 28-37 amino acids, they are characterized by their head-to-tail cyclised peptide backbone and the interlocking arrangement of their three ...
s'' are RiPPs with a head-to-tail cyclization and three conserved disulfide bonds
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In in ...
that form a knotted structure called a cyclic cysteine knot motif. No other posttranslational modifications have been observed on the characterized cyclotides, which are between 28 - 37 amino acids in size. Cyclotides are plant natural products and the different cyclotides appear to be species-specific. While many activities have been reported for cyclotides, it has been hypothesized that all are united by a common mechanism of binding to and disrupting the cell membrane.
'' Glycocins'' are RiPPs that are glycosylated
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not ...
antimicrobial peptides
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between Prokaryote, prokaryotic and eukaryota, eukaryotic cells that may ...
. Only two members have been fully characterized, making this a small RiPP class. Sublancin 168 and glycocin F are both Cys-glycosylated and, in addition, have disulfide bonds between non-glycosylated Cys residues. While both members bear S-glycosyl groups, RiPPs bearing O- or N-linked carbohydrates will also be included in this family as they are discovered.
''Linaridins'' are characterized by C-terminal aminovinyl cysteine residues. While this posttranslational modification is also seen in the lanthipeptides epidermin and mersacidin, linaridins do not have Lan or MeLan residues. In addition, the linaridin moiety is formed from modification of two Cys residues, whereas lanthipeptide aminovinyl cysteines are formed from Cys and dehydroalanine
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As ...
(Dha). The first linaridin to be characterized was cypemycin.
'' Microviridins'' are cyclic ''N''-acetylated trideca- and tetradecapeptides with ω-ester and/or ω-amide bonds. Lactone formation through glutamate or aspartate ω-carboxy groups and the lysine ε-amino group forms macrocycles in the final natural product. This class of RiPPs function as protease inhibitors
Protease inhibitors (PIs) are medications that act by interfering with protease, enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS, hepatitis C and COVID-19. These protease inhibitors pre ...
and were originally isolated from ''Microcystis
''Microcystis'' is a genus of freshwater cyanobacteria that includes the harmful algal bloom-forming '' Microcystis aeruginosa''.
Over the last few decades, cyanobacterial blooms caused by eutrophication have become a major environmental proble ...
viridis''. Gene clusters encoding microviridins have also been identified in genomes across the Bacteroidetes
The phylum Bacteroidota (synonym Bacteroidetes) is composed of three large classes of Gram-negative, nonsporeforming, anaerobic or aerobic, and rod-shaped bacteria that are widely distributed in the environment, including in soil, sediments, and ...
and Proteobacteria
Pseudomonadota (synonym "Proteobacteria") is a major phylum of gram-negative bacteria. Currently, they are considered the predominant phylum within the domain of bacteria. They are naturally found as pathogenic and free-living (non-parasitic) ...
phyla.
''Orbitides'' are plant-derived N-to-C cyclized peptides with no disulfide bonds. Also referred to as Caryophyllaceae-like homomonocyclopeptides, orbitides are 5-12 amino acids in length and are composed of mainly hydrophobic residues. Similar to the amatoxins and phallotoxins, the gene sequences of orbitides suggest the presence of a C-terminal recognition sequence. In the flaxseed variety ''Linum usitatissimum'', a precursor peptide was found using Blast searching that potentially contains five core peptides separated by putative recognition sequences.
''Proteusins'' are named after "Proteus", a Greek shape-shifting sea god. Until now, the only known members in the family of Proteusins are called polytheonamides. They were originally presumed to be Nonribosomal peptide, nonribosomal natural products due to the presence of many D-amino acids and other non-proteinogenic amino acids. However, a metagenomic study revealed the natural products as the most extensively modified class of RiPPs known to date. Six enzymes are responsible for installing a total of 48 posttranslational modifications onto the polytheonamide A and B precursor peptides, including 18 epimerizations. Polytheonamides are exceptionally large, as a single molecule is able to span a cell membrane and form an ion channel.
''Sactipeptides'' contain intramolecular linkages between the sulfur of Cys residues and the α-carbon of another residue in the peptide. A number of nonribosomal peptide
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacterium, bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be ma ...
s bear the same modification. In 2003, the first RiPP with a sulfur-to-α-carbon linkage was reported when the structure of subtilosin A was determined using isotopically enriched media and NMR spectroscopy. In the case of subtilosin A, isolated from Bacillus subtilis, Bacillus subtilis 168, the Cα crosslinks between Cys4 and Phe31, Cys7 and Thr28, and Cys13 and Phe22 are not the only posttranslational modifications; the C- and N-termini form an amide bond, resulting in a circular structure that is conformationally restricted by the Cα bonds. Sactipeptides with antimicrobial activity are commonly referred to as sactibiotics (''s''ulfur to ''a''lpha-''c''arbon an''tibiotic'').
Biosynthesis
RiPPs are characterized by a common biosynthetic strategy wherein genetically-encoded peptides undergo translation and subsequent chemical modification by biosynthetic enzymes.Common features
 All RiPPs are synthesized first at the ribosome as a precursor peptide. This peptide consists of a core peptide segment which is typically preceded (and occasionally followed) by a leader peptide segment and is typically ~20-110 residue (chemistry), residues long. The leader peptide is usually important for enabling enzymatic processing of the precursor peptide via aiding in recognition of the core peptide by biosynthetic enzymes and for cellular export. Some RiPPs also contain a recognition sequence C-terminal to the core peptide; these are involved in excision and cyclization. Additionally, eukaryotic RiPPs may contain a signal segment of the precursor peptide which helps direct the peptide to cellular compartments.
During RiPP biosynthesis, the unmodified precursor peptide (containing an unmodified core peptide, UCP) is recognized and chemically modified sequentially by biosynthetic enzymes (PRPS). Examples of modifications include dehydration (i.e. lantibiotics, lanthipeptides, thiopeptides), cyclodehydration (i.e. thiopeptides), prenylation (i.e. cyanobactins), and cyclization (i.e. lasso peptides), among others. The resulting modified precursor peptide (containing a modified core peptide, MCP) then undergoes proteolysis, wherein the non-core regions of the precursor peptide are removed. This results in the mature RiPP.
All RiPPs are synthesized first at the ribosome as a precursor peptide. This peptide consists of a core peptide segment which is typically preceded (and occasionally followed) by a leader peptide segment and is typically ~20-110 residue (chemistry), residues long. The leader peptide is usually important for enabling enzymatic processing of the precursor peptide via aiding in recognition of the core peptide by biosynthetic enzymes and for cellular export. Some RiPPs also contain a recognition sequence C-terminal to the core peptide; these are involved in excision and cyclization. Additionally, eukaryotic RiPPs may contain a signal segment of the precursor peptide which helps direct the peptide to cellular compartments.
During RiPP biosynthesis, the unmodified precursor peptide (containing an unmodified core peptide, UCP) is recognized and chemically modified sequentially by biosynthetic enzymes (PRPS). Examples of modifications include dehydration (i.e. lantibiotics, lanthipeptides, thiopeptides), cyclodehydration (i.e. thiopeptides), prenylation (i.e. cyanobactins), and cyclization (i.e. lasso peptides), among others. The resulting modified precursor peptide (containing a modified core peptide, MCP) then undergoes proteolysis, wherein the non-core regions of the precursor peptide are removed. This results in the mature RiPP.
Nomenclature
Papers published prior to a recent community consensus employ differing sets of nomenclature. The precursor peptide has been referred to previously as ''prepeptide'', ''prepropeptide'', or ''structural peptide''. The leader peptide has been referred to as a ''propeptide'', ''pro-region'', or ''intervening region''. Historical alternate terms for core peptide included ''propeptide'', ''structural peptide'', and ''toxin region'' (for conopeptides, specifically).Family-specific features
Lanthipeptides
Lanthipeptides are characterized by the presencelanthionine
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element ...
(Lan) and 3-methyllanthionine (MeLan) residues. Lan residues are formed from a thioether bridge between Cys and Ser, while MeLan residues are formed from the linkage of Cys to a Thr residue. The biosynthetic enzymes responsible for Lan and MeLan installation first dehydrate Ser and Thr to dehydroalanine
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As ...
(Dha) and dehydrobutyrine (Dhb), respectively. Subsequent thioether crosslinking occurs through a Michael addition, Michael-type addition by Cys onto Dha or Dhb.
Four classes of lanthipeptide biosynthetic enzymes have been designated. Class I lanthipeptides have dedicated lanthipeptide dehydratases, called LanB enzymes, though more specific designations are used for particular lanthipeptides (e.g. NisB is the nisin dehydratase). A separate cyclase, LanC, is responsible for the second step in Lan and MeLan biosynthesis. However, class II, III, and IV lanthipeptides have bifunctional lanthionine synthetases in their gene clusters, meaning a single enzyme carries out both dehydration and cyclization steps. Class II synthetases, designated LanM synthetases, have N-terminal dehydration domains with no sequence homology to other lanthipeptide biosynthetic enzymes; the cyclase domain has homology to LanC. Class III (LanKC) and IV (LanL) enzymes have similar N-terminal lyase and central kinase domains, but diverge in C-terminal cyclization domains: the LanL cyclase domain is homologous to LanC, but the class III enzymes lack Zn-ligand binding domains.
Linear azol(in)e-containing peptides
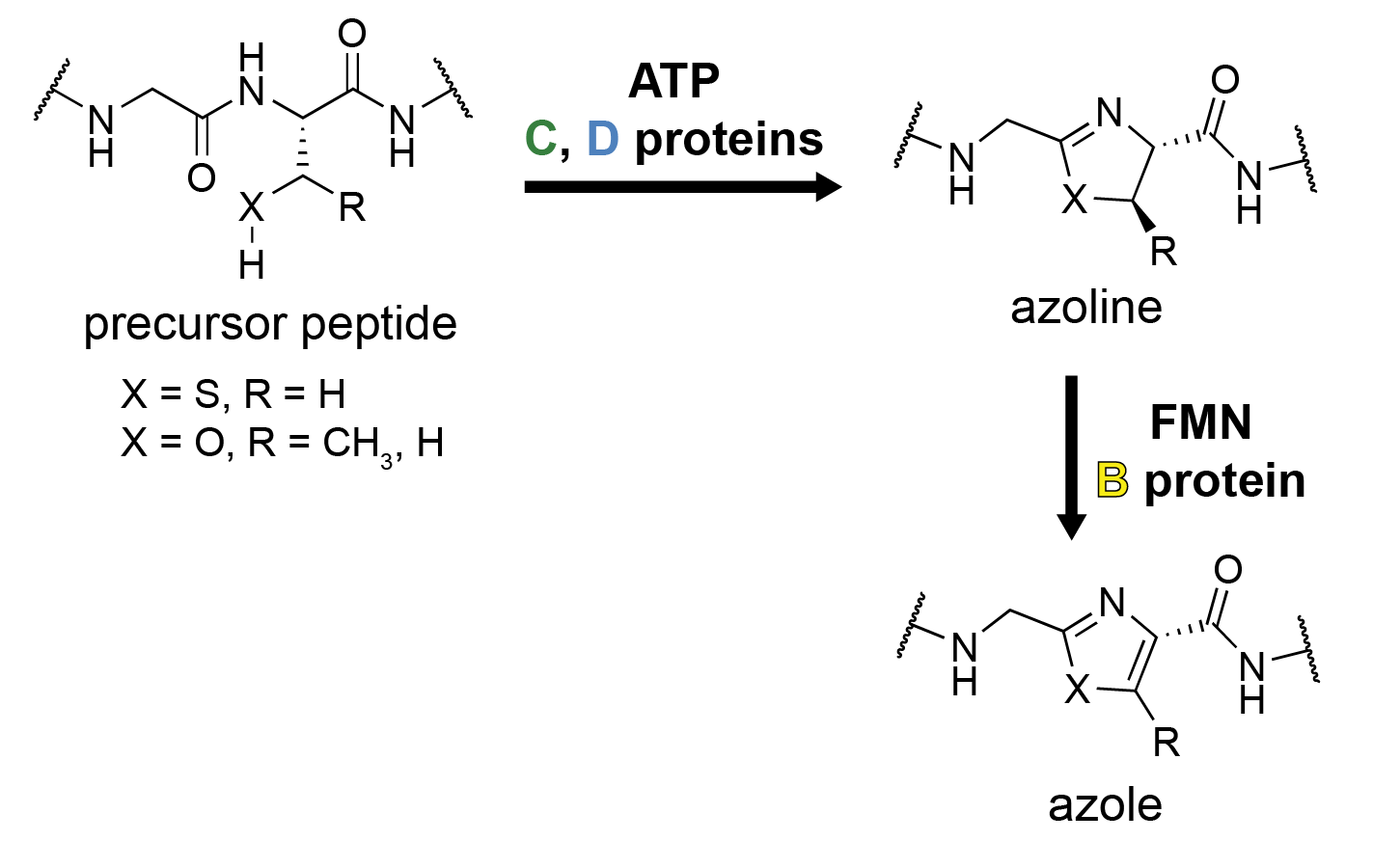
 The hallmark of linear azol(in)e-containing peptide (LAP) biosynthesis is the formation of azole, azol(in)e heterocyclic compound, heterocycles from the nucleophile, nucleophilic amino acids
The hallmark of linear azol(in)e-containing peptide (LAP) biosynthesis is the formation of azole, azol(in)e heterocyclic compound, heterocycles from the nucleophile, nucleophilic amino acids serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
, threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− ...
, or cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
. This is accomplished by three enzymes referred to as the B, C, and D proteins; the precursor peptide is referred to as the A protein, as in other classes.
The C protein is mainly involved in leader peptide recognition and binding and is sometimes called a scaffolding protein. The D protein is an ATP-dependent cyclodehydratase that catalyzes the cyclodehydration reaction, resulting in formation of an azoline ring. This occurs by direct activation of the amide backbone carbonyl with ATP, resulting in stoichiometric ATP consumption. The C and D proteins are occasionally present as a single, fused protein, as is the case for trunkamide biosynthesis. The B protein is a flavin mononucleotide (FMN)-dependent dehydrogenase which oxidation, oxidizes certain azoline rings into azoles.
The B protein is typically referred to as the dehydrogenase; the C and D proteins together form the cyclodehydratase, although the D protein alone performs the cyclodehydration reaction. Early work on microcin B17 adopted a different nomenclature for these proteins, but a recent consensus has been adopted by the field as described above.
Cyanobactins
Cyanobactin biosynthesis requires proteolytic cleavage of both N-terminal and C-terminal portions of the precursor peptide. The defining proteins are thus an N-terminalprotease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
, referred to as the A protein, and a C-terminal protease, referred to as the G protein. The G protein is also responsible for macrocycle, macrocyclization.
For cyanobactins, the precursor peptide is referred to as the E peptide. Minimally, the E peptide requires a leader peptide region, a core (structural) region, and both N-terminal and C-terminal protease recognition sequences. In contrast to most RiPPs, for which a single precursor peptide encodes a single natural product via a lone core peptide, cyanobactin E peptides can contain multiple core regions; multiple E peptides can even be present in a single gene cluster.
Many cyanobactins also undergo heterocyclization by a heterocyclase (referred to as the D protein), installing oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the ...
or thiazoline
Thiazolines (; or dihydrothiazoles) are a group of isomeric 5-membered heterocyclic compounds containing both sulfur and nitrogen in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivative (chemistry), der ...
moieties from Ser/Thr/Cys residues prior to the action of the A and G proteases. The heterocyclase is an adenosine triphosphate, ATP-dependent YcaO Sequence homology, homologue that behaves biochemically in the same manner as YcaO-domain cyclodehydratases in thiopeptide and linear azol(in)e-containing peptide (LAP) biosynthesis (described above).
A common modification is prenylation of hydroxyl groups by an F protein prenyltransferase. Oxidation of azoline heterocycles to azoles can also be accomplished by an oxidase protein domain, domain located on the G protein. Unusual for ribosome, ribosomal peptides, cyanobactins can include D-amino acids; these can occur adjacent to azole or azoline residues. The functions of some proteins found commonly in cyanobactin biosynthetic gene clusters, the B and C proteins, are unknown.
Thiopeptides
Thiopeptide biosynthesis involves particularly extensive modification of the core peptide scaffold. Indeed, due to the highly complex structures of thiopeptides, it was commonly thought that thesenatural products
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
were nonribosomal peptide
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacterium, bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be ma ...
s. Recognition of the ribosome, ribosomal origin of these molecules came in 2009 with the independent discovery of the gene clusters for several thiopeptides.
The standard nomenclature for thiopeptide biosynthetic proteins follows that of the thiomuracin gene cluster. In addition to the precursor peptide, referred to as the A peptide, thiopeptide biosynthesis requires at least six genes. These include lanthipeptide-like dehydratases, designated the B and C proteins, which install dehydroalanine
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As ...
and dehydrobutyrine moieties by dehydrating Ser/Thr precursor residues. Azole and azoline synthesis is effected by the E protein, the dehydrogenase
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as ...
, and the G protein, the cyclodehydratase. The nitrogen-containing heterocycle is installed by the D protein cyclase via a putative [4+2] cycloaddition of dehydroalanine moieties to form the characteristic macrocycle. The F protein is responsible for binding of the leader peptide.
Thiopeptide biosynthesis is biochemically similar to that of cyanobactins, lanthipeptides, and linear azol(in)e-containing peptides (LAPs). As with cyanobactins and LAPs, azole and azoline synthesis occurs via the action of an adenosine triphosphate, ATP-dependent YcaO-protein domain, domain cyclodehydratase. In contrast to LAPs, where cyclodehydration occurs via the action of two distinct proteins responsible for leader peptide binding and cyclodehydrative catalysis, these are fused into a single protein (G protein) in cyanobactin and thiopeptide biosynthesis. However, in thiopeptides, an additional protein, designated the Ocin-ThiF-like protein (F protein) is necessary for leader peptide recognition and potentially recruiting other biosynthetic enzymes.
Lasso peptides
 Lasso peptide biosynthesis requires at least three genes, referred to as the A, B, and C proteins. The A gene encodes the precursor peptide, which is modified by the B and C proteins into the mature natural product. The B protein is an adenosine triphosphate-dependent cysteine protease that cleaves the leader region from the precursor peptide. The C protein displays Homologous series, homology to asparagine synthetase and is thought to activate the carboxylic acid side chain of a
Lasso peptide biosynthesis requires at least three genes, referred to as the A, B, and C proteins. The A gene encodes the precursor peptide, which is modified by the B and C proteins into the mature natural product. The B protein is an adenosine triphosphate-dependent cysteine protease that cleaves the leader region from the precursor peptide. The C protein displays Homologous series, homology to asparagine synthetase and is thought to activate the carboxylic acid side chain of a glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that ...
or aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protein ...
residue via adenylate, adenylylation. The N-terminal amine formed by the B protein (protease) then reacts with this activated side chain to form the macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
-forming isopeptide bond. The exact steps and reaction intermediates in lasso peptide biosynthesis remain unknown due to experimental difficulties associated with the proteins. Commonly, the B protein is referred to as the lasso protease, and the C protein is referred to as the lasso cyclase.
Some lasso peptide biosynthetic gene clusters also require an additional protein of unknown function for biosynthesis. Additionally, lasso peptide gene clusters usually include an ATP-binding cassette transporter, ABC transporter (D protein) or an isopeptidase, although these are not strictly required for lasso peptide biosynthesis and are sometimes absent. No crystal structure, X-ray crystal structure is yet known for any lasso peptide biosynthetic protein.
The biosynthesis of lasso peptides is particularly interesting due to the inaccessibility of the threaded-lasso topology
Topology (from the Greek language, Greek words , and ) is the branch of mathematics concerned with the properties of a Mathematical object, geometric object that are preserved under Continuous function, continuous Deformation theory, deformat ...
to chemical peptide synthesis.
See also
* Nonribosomal peptideReferences
{{reflist Biosynthesis Molecular biology Enzymes Peptides