Ionic Hydrogenations on:
[Wikipedia]
[Google]
[Amazon]
Ionic hydrogenation refers to
 Upon the generation of a carbocation, rate-determining hydride transfer from the organosilane occurs to yield a reduced product. Retention of configuration at silicon has been observed in silane reductions of chiral triaryl methyl chlorides in benzene. This result suggests that the exchange of chlorine for hydrogen occurs through σ-bond metathesis. Reductions in more polar solvents may involve silicenium ions.
Upon the generation of a carbocation, rate-determining hydride transfer from the organosilane occurs to yield a reduced product. Retention of configuration at silicon has been observed in silane reductions of chiral triaryl methyl chlorides in benzene. This result suggests that the exchange of chlorine for hydrogen occurs through σ-bond metathesis. Reductions in more polar solvents may involve silicenium ions.
 Polymeric
Polymeric 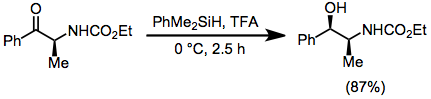
hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
achieved by the addition of a hydride to substrate that has been activated by an electrophile. Some ionic hydrogenations entail addition of H2 to the substrate and some entail replacement of a heteroatom with hydride. Traditionally, the method was developed for acid-induced reductions with hydrosilane
Hydrosilanes are tetravalent silicon compounds containing one or more Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Examples include phenylsilane (PhSiH3) and triethoxysilane ((C2H ...
s. Alternatively ionic hydrogenation can be achieved using H2. Bullock, R. M. "Ionic Hydrogenations," in The Handbook of Homogeneous Hydrogenation (eds J. G. de Vries and C. J. Elsevier), Wiley-VCH Verlag GmbH, Weinheim, Germany, 2007. Ionic hydrogenation is employed when the substrate can produce a stable carbonium ion
In chemistry, a carbonium ion is a cation that has a pentacoordinated carbon atom. They are a type of carbocation. In older literature, the name "carbonium ion" was used for what is today called carbenium. Carbonium ions charge is delocalized ...
. Polar double bonds are favored substrates.
Using hydrosilanes
Because silicon (1.90) is more electropositive than hydrogen (2.20), hydrosilanes exhibit (mild) hydridic character. Hydrosilanes can serve as hydride donors to highly electrophilic organic substrates. Many alcohols, alkyl halides, acetals, orthoesters, alkenes, aldehydes, ketones, and carboxylic acid derivatives are suitable substrates. Such reactions often requireLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
s. Only reactive electrophiles undergo reduction, selectivity is possible in reactions of substrates with multiple reducible functional groups.
 Upon the generation of a carbocation, rate-determining hydride transfer from the organosilane occurs to yield a reduced product. Retention of configuration at silicon has been observed in silane reductions of chiral triaryl methyl chlorides in benzene. This result suggests that the exchange of chlorine for hydrogen occurs through σ-bond metathesis. Reductions in more polar solvents may involve silicenium ions.
Upon the generation of a carbocation, rate-determining hydride transfer from the organosilane occurs to yield a reduced product. Retention of configuration at silicon has been observed in silane reductions of chiral triaryl methyl chlorides in benzene. This result suggests that the exchange of chlorine for hydrogen occurs through σ-bond metathesis. Reductions in more polar solvents may involve silicenium ions.
 Polymeric
Polymeric hydrosilanes
Hydrosilanes are tetravalent silicon compounds containing one or more Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Examples include phenylsilane (PhSiH3) and triethoxysilane ((C2H5 ...
, such as polymethylhydrosiloxane (PHMS) may be employed to facilitate separation of the reduced products from silicon-containing byproducts.
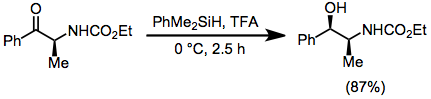
Using H2
The proton and hydride transfers are usually sequential or concerted. Usually ionic hydrogenation is shown to occur in two steps, starting with protonation. :R2C=Y + H+ → R2C+-YH :R2C+-YH + "H−" → R2CH-YHSubstrates
In the case of metal-catalyzed ionic hydrogenation, the substrates and their products must not bind to metal sites, as this would interfere with H2 activation.Ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
are the most common substrates. Less common are imines and N-heterocycles. The reaction can also be performed in reverse to effect hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
. Liquid substrates can sometimes be hydrogenated without solvent, a goal of green chemistry
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. Wh ...
.
Proton and hydride pairs
The most common hydrogenating pair is an organosilane as the hydride source (e.g.triethylsilane
Triethylsilane is the organosilicon compound with the formula (C2H5)3SiH. It is a trialkylsilane. The Si-H bond is reactive.
It was first discovered by Albert Ladenburg in 1872 among the products of reduction of tetraethyl orthosilicate with sod ...
), and a strong oxyacid as the proton source (e.g. trifluoroacetic acid
Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not ...
or triflic acid
Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for este ...
). The hydride and proton source cannot combine to give H2, which limits the hydricity and acidity
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the ...
of the H− and H+ sources, respectively.
Transition metal hydride
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loose ...
complexes can be used in place of organosilanes as the hydride source. In these cases, triflic acid is a typical proton donor. Ketones such as benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and ros ...
s, and 1,1-disubstituted olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
s are typical substrates. Hydrides of tungsten, chromium, osmium, and molybdenum complexes have also been reported. Tungsten dihydride complexes can hydrogenate ketones stoichiometrically with no external acids. One hydride serves as the hydride source, and the other serves as a proton source.
In the case of ionic hydrogenation, a dihydride complex is regenerated by hydrogen gas following hydrogenation. Typical catalysts are tungsten or molybdenum complexes. An example of such a catalyst is CpMo(CO)2(PR3)(OCR'2)]+ where M = W or Mo.
Related reactions
Transfer hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and re ...
(TH) catalysts, e.g. Shvo catalyst
The Shvo catalyst is an organoruthenium compound that catalyzes the hydrogenation of polar functional groups including aldehydes, ketones and imines. The compound is of academic interest as an early example of a catalyst for transfer hydrogenation ...
, are related to catalysts used for ionic hydrogenation. TH catalysts however do not employ strong acids and both the H− and H+ components are covalently bonded to the complex prior to transfer to the unsaturated substrates. Typically, TH catalysts are more widely employed in organic synthesis.Eisenstein, O.; Crabtree, R. H., "Outer sphere hydrogenation catalysis", New J. Chem. 2013, vol. 37, 21
Older literature
*{{cite journal, last1=Kursanov, first1=D. N., last2=Parnes, first2=Z. N., last3=Loim, first3=N. M., title=Applications of Ionic Hydrogenation to Organic Synthesis, journal=Synthesis, year=1974 , volume=1974, issue=9, pages=633–651, doi=10.1055/s-1974-23387References