iodoform test on:
[Wikipedia]
[Google]
[Amazon]
In 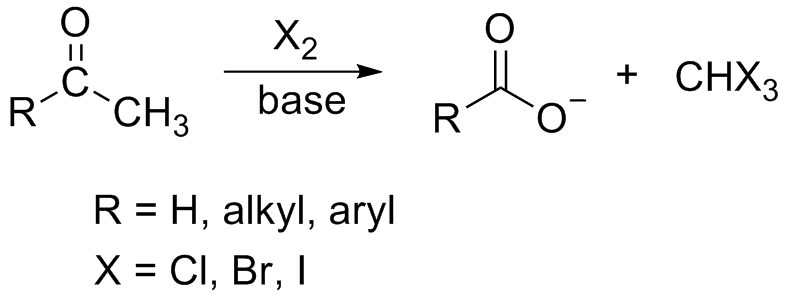
Br2 + 2 OH- -> Br- + BrO- + H2O
If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite:
If a methyl ketone is present, it reacts with the hypohalite in a three-step process:
1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge).
: 2. When the α(alpha) position has been exhaustively halogenated, the molecule undergoes a nucleophilic acyl substitution by hydroxide, with being the leaving group stabilized by three
2. When the α(alpha) position has been exhaustively halogenated, the molecule undergoes a nucleophilic acyl substitution by hydroxide, with being the leaving group stabilized by three 
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, the haloform reaction is a chemical reaction in which a haloform (, where X is a halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
) is produced by the exhaustive halogenation
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, ...
of an acetyl group
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, ...
(, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups () or to produce chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to ...
(), bromoform (), or iodoform (). Note that fluoroform () can't be prepared in this way.

Mechanism
In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite. :electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
s. In the third step the anion abstracts a proton from either the solvent or the carboxylic acid formed in the previous step, and forms the haloform. At least in some cases ( chloral hydrate) the reaction may stop and the intermediate product isolated if conditions are acidic and hypohalite is used.
:Scope
Substrates are broadly limited to methyl ketones and secondaryalcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s oxidizable to methyl ketones, such as isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
. The only primary alcohol and aldehyde to undergo this reaction are ethanol and acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the mos ...
, respectively. 1,3-Diketones such as acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are tre ...
also give the haloform reaction. β-ketoacids such as acetoacetic acid will also give the test upon heating. Acetyl chloride and acetamide don't give this test. The halogen used may be chlorine, bromine, iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
or sodium hypochlorite. Fluoroform (CHF3) cannot be prepared by this method as it would require the presence of the highly unstable hypofluorite
Hypofluorous acid, chemical formula H O F, is the only known oxyacid of fluorine and the only known oxoacid in which the main atom gains electrons from oxygen to create a negative oxidation state. The oxidation state of the oxygen in hypofluorite ...
ion. However ketones with the structure RCOCF3 do cleave upon treatment with base to produce fluoroform; this is equivalent to the second and third steps in the process shown above.
Applications
Laboratory scale
This reaction forms the basis of the iodoform test which was commonly used in history as a chemical test to determine the presence of a methyl ketone, or a secondary alcohol oxidizable to a methyl ketone. When iodine and sodium hydroxide are used as the reagents a positive reaction gives iodoform, which is a solid at room temperature and tends to precipitate out of solution causing a distinctive cloudiness. In organic chemistry, this reaction may be used to convert a terminal methyl ketone into the analogous carboxylic acid.Industrially
It was formerly used to produce iodoform, bromoform, and even chloroform industrially.As a by-product of water chlorination
Water chlorination can result in the formation of haloforms if the water contains suitable reactive impurities (e.g.humic acid Humic substances (HS) are organic compounds that are important components of humus, the major organic fraction of soil, peat, and coal (and also a constituent of many upland streams, dystrophic lakes, and ocean water). For a long era in the 19th ...
). There is a concern that such reactions may lead to the presence of carcinogenic compounds in drinking water.
History
The haloform reaction is one of the oldestorganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
s known. In 1822, Georges-Simon Serullas added potassium metal to a solution of iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
in ethanol and water to form potassium formate and iodoform, called in the language of that time ''hydroiodide of carbon''. In 1832, Justus von Liebig
Justus Freiherr von Liebig (12 May 1803 – 20 April 1873) was a German scientist who made major contributions to agricultural and biological chemistry, and is considered one of the principal founders of organic chemistry. As a professor at t ...
reported the reaction of chloral with calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has m ...
to form chloroform and calcium formate. The reaction was rediscovered by Adolf Lieben
Adolf Lieben (3 December 1836 – 6 June 1914) was an Austrian Jewish chemist. He was born in Vienna the son of Ignatz Lieben. He studied at the University of Vienna, University of Heidelberg (Ph.D. 1856 with Robert Wilhelm Bunsen), and Paris ...
in 1870.See:
*
* The iodoform test is also called the Lieben haloform reaction. A review of the haloform reaction with a history section was published in 1934.
References
{{Reflist, 2 Organic redox reactions Carbon-heteroatom bond forming reactions Halogenation reactions