Insertion Reaction on:
[Wikipedia]
[Google]
[Amazon]
An insertion reaction is a
 Mechanistically, the α-diazoketone undergoes a
Mechanistically, the α-diazoketone undergoes a  Perhaps surprisingly,
Perhaps surprisingly,  The
The 


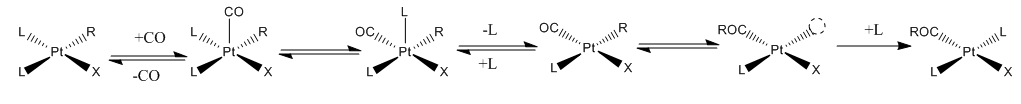 Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but
Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but  The Cativa process
The Cativa process

chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
where one chemical entity (a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
or molecular fragment) interposes itself into an existing bond
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Fidelity bond, a type of insurance policy for employers
* Chemical bond, t ...
of typically a second chemical entity ''e.g.'':
:
The term only refers to the result of the reaction and does not suggest a mechanism
Mechanism may refer to:
*Mechanism (economics), a set of rules for a game designed to achieve a certain outcome
**Mechanism design, the study of such mechanisms
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a ...
. Insertion reactions are observed in organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
, inorganic
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''.
Inor ...
, and organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
chemistry. In cases where a metal-ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
bond in a coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
is involved, these reactions are typically organometallic in nature and involve a bond between a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
and a carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
or hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
. It is usually reserved for the case where the coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
and oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
of the metal remain unchanged. When these reactions are reversible, the removal of the small molecule from the metal-ligand bond is called extrusion or elimination.
There are two common insertion geometries— 1,1 and 1,2 (pictured above). Additionally, the inserting molecule can act either as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
or as an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
to the metal complex. These behaviors will be discussed in more detail for CO, nucleophilic behavior, and SO2, electrophilic behavior.
Organic chemistry
Homologation reaction
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant ...
s like the Kowalski ester homologation The Kowalski ester homologation is a chemical reaction for the homologation reaction, homologation of esters.
This reaction was designed as a safer alternative to the Arndt–Eistert synthesis, avoiding the need for diazomethane. The Kowalski rea ...
provide simple examples of insertion process in organic synthesis. In the Arndt-Eistert reaction, a methylene unit is inserted into the carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ...
-carbon bond of carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
to form the next acid in the homologous series
In organic chemistry, a homologous series is a sequence of compounds with the same functional group and similar chemical properties in which the members of the series differ by the number of repeating units they contain. This can be the length of ...
. ''Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
'' provides the example of ''t''-BOC protected (''S'')-phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the chemical formula, formula . It can be viewed as a benzyl group substituent, substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of ...
(2-amino-3-phenylpropanoic acid) being reacted sequentially with triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
, ethyl chloroformate
Ethyl chloroformate is an organic compound with the chemical formula . It is the ethyl ester of chloroformic acid. It is a colorless, corrosive and highly toxic liquid. It is a reagent used in organic synthesis for the introduction of the ethyl c ...
, and diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
to produce the α-diazoketone, which is then reacted with silver trifluoroacetate / triethylamine in aqueous solution to generate the ''t''-BOC protected form of (''S'')-3-amino-4-phenylbutanoic acid.
: Mechanistically, the α-diazoketone undergoes a
Mechanistically, the α-diazoketone undergoes a Wolff rearrangement
The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate prod ...
to form a ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na ...
in a 1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Frank C. Whitmore, Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atom ...
. Consequently, the methylene group α- to the carboxyl group in the product is the methylene group from the diazomethane reagent. The 1,2-rearrangement has been shown to conserve the stereochemistry of the chiral centre as the product formed from ''t''-BOC protected (''S'')-phenylalanine retains the (''S'') stereochemistry with a reported enantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a sing ...
of at least 99%.
A related transformation is the Nierenstein reaction in which a diazomethane methylene group is inserted into the carbon-chlorine bond of an acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
to generate an α-chloro ketone. An example, published in 1924, illustrates the reaction in a substituted benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula . It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring () with an acyl chloride () substituent. It is ...
system:
: Perhaps surprisingly,
Perhaps surprisingly, α-bromoacetophenone
Phenacyl bromide is the organic compound with the chemical formula, formula C6H5C(O)CH2Br. This colourless solid is a powerful lachrymatory agent, lachrymator as well as a useful precursor to other organic compounds.
It is prepared by brominatio ...
is the minor product when this reaction is carried out with benzoyl bromide
In organic chemistry, benzoyl (, ) is the functional group with the Chemical formula, formula and Chemical structure, structure . It can be viewed as benzaldehyde missing one hydrogen. The benzoyl group has a mass of 105 amu.
The term "benzoy ...
, a dimeric dioxane
Dioxane may refer to the following chemical compounds:
* 1,2-dioxane
* 1,3-dioxane
* 1,4-dioxane
{{Authority control ...
being the major product. Organic azides also provide an example of an insertion reaction in organic synthesis and, like the above examples, the transformations proceed with loss of nitrogen gas
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh i ...
. When tosyl azide
Tosyl azide is a reagent used in organic synthesis.
Uses
Tosyl azide is used for the introduction of azide and diazo functional groups. It is also used as a nitrene source and as a substrate for +2cycloaddition reactions.
Preparation
Tosyl az ...
reacts with norbornadiene
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distin ...
, a ring expansion reaction takes place in which a nitrogen atom is inserted into a carbon-carbon bond α- to the bridge head:
: The
The Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement reaction, rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on ...
is another example of a ring expanding reaction in which a heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
is inserted into a carbon-carbon bond. The most important application of this reaction is the conversion of cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
to its oxime, which is then rearranged under acidic conditions to provide ε-caprolactam
Caprolactam (CPL) is an organic compound with the chemical formula, formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast ...
, the feedstock for the manufacture of Nylon 6
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the compari ...
. Annual production of caprolactam exceeds 2 billion kilograms.
:Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s undergo both intermolecular
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
and intramolecular insertion reactions. Cyclopentene
Cyclopentene is a chemical compound with the formula . It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of t ...
moieties can be generated from sufficiently long-chain ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s by reaction with trimethylsilyldiazomethane
Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is commercially available as solutions in hexanes, DCM, and ether. It is a specialized reag ...
, (CH3)3Si–CHN2:
:
Here, the carbene intermediate inserts into a carbon-hydrogen bond to form the carbon-carbon bond needed to close the cyclopentene ring. Carbene insertions into carbon-hydrogen bonds can also occur intermolecularly:
:Carbenoid In chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons–Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of
:I-CH2-Zn-I
This complex reacts w ...
s are reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s that behave similarly to carbenes. One example is the chloroalkyllithium carbenoid reagent prepared ''in situ'' from a sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
and ''t''-BuLi which inserts into the carbon-boron bond of a pinacol boronic ester
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
:
:
Organometallic chemistry
Many reactions in organometallic chemistry involve insertion of one ligand (L) into a metal-hydride or metal-alkyl/aryl bond. Generally it is the hydride, alkyl, oraryl group
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
that migrates onto L, which is often CO, an alkene, or alkyne.
Carbonylations
The insertion of carbon monoxide and alkenes into metal-carbon bonds is a widely exploited reaction with major industrial applications. Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but
Such reactions are subject to the usual parameters that affect other reactions in coordination chemistry, but steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
are especially important in determining the stereochemistry and regiochemistry of the reactions. The reverse reaction, the de-insertion of CO and alkenes, are of fundamental significance in many catalytic cycles as well.
Widely employed applications of migratory insertion
In organometallic chemistry, a migratory insertion is a type of chemical reaction, reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiate ...
of carbonyl groups are hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
and the carbonylative production of acetic acid. The former converts alkenes, hydrogen, and carbon monoxide into aldehydes. The production of acetic acid by carbonylation proceeds via two similar industrial processes. More traditional is the rhodium-based Monsanto acetic acid process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chemic ...
, but this process has been superseded by the iridium-based Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an ...
. By 2002, worldwide annual production of acetic acid stood at 6 million tons, of which approximately 60% is produced by the Cativa process.
: The Cativa process
The Cativa process catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
, shown above, includes both insertion and de-insertion steps. The oxidative addition reaction of methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h ...
with (1) involves the formal insertion of the iridium(I) centre into the carbon-iodine bond, whereas step (3) to (4) is an example of migratory insertion of carbon monoxide into the iridium-carbon bond. The active catalyst species is regenerated by the reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
of acetyl iodide
Acetyl iodide is an organoiodine compound with the formula CH3COI. It is a colourless liquid. It is formally derived from acetic acid. Although far rarer in the laboratory than the related acetyl bromide and acetyl chloride, acetyl iodide is pr ...
from (4), a de-insertion reaction.
Olefin insertion
The insertion of ethylene and propylene into titanium alkyls is the cornerstone of Ziegler-Natta catalysis, the commercial route of polyethylene and polypropylene. This technology mainly involves heterogeneous catalysts, but it is widely assumed that the principles and observations on homogeneous systems are applicable to the solid-state versions. Related technologies include theShell Higher Olefin Process
The Shell higher olefin process (SHOP) is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Shell plc.''Industrial Organic Chemistry'', Klaus Weissermel, Hans ...
which produces detergent
A detergent is a surfactant or a mixture of surfactants with Cleanliness, cleansing properties when in Concentration, dilute Solution (chemistry), solutions. There are a large variety of detergents. A common family is the alkylbenzene sulfonate ...
precursors. the olefin can be coordinated to the metal before insertion. Depending on the ligand density of the metal, ligand dissociation may be necessary to provide a coordination site for the olefin.
Other insertion reactions in coordination chemistry
Many electrophilic oxides insert into metal carbon bonds; these includesulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
, carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, and nitric oxide. These reactions have limited practical significance, but are of historic interest. With transition metal alkyls, these oxides behave as electrophiles and insert into the bond between metals and their relatively nucleophilic alkyl ligands. As discussed in the article on Metal sulfur dioxide complex
In organometallic chemistry, metal sulfur dioxide complexes are complexes that contain sulfur dioxide, , bonded to a transition metal. Such compounds are common but are mainly of theoretical interest. Historically, the study of these compounds ...
es, the insertion of SO2 has been examined in particular detail.
More insertion reactions in organic chemistry
Electropositive metals such as sodium, potassium, magnesium, zinc, etc. can insert into alkyl halides, breaking the carbon-halide bond ( halide could be chlorine, bromine, iodine ) and forming a carbon-metal bond. This reaction happens via a SET mechanism ( single-electron-transfer mechanism ). If magnesium reacts with an alkyl halide, it forms aGrignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
, or if lithium reacts, an organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
is formed. Thus, this type of insertion reactions has important applications in chemical synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses ...
.

References
{{DEFAULTSORT:Insertion Reaction Organometallic chemistry