homoaromaticity on:
[Wikipedia]
[Google]
[Amazon]
Homoaromaticity, in  The concept of homoaromaticity was pioneered by
The concept of homoaromaticity was pioneered by
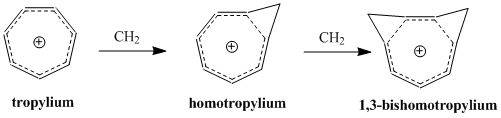
 In a series of acetolysis experiments, Winstein et al. observed that the
In a series of acetolysis experiments, Winstein et al. observed that the
 From this observation, Pettit, et al. concluded that the classical structure of the cyclooctatrienyl cation must be incorrect. Instead, the group proposed the structure of the bicyclo .1.0ctadienyl compound, theorizing that the
From this observation, Pettit, et al. concluded that the classical structure of the cyclooctatrienyl cation must be incorrect. Instead, the group proposed the structure of the bicyclo .1.0ctadienyl compound, theorizing that the 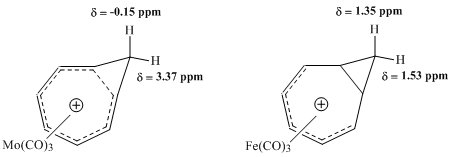 Subsequent NMR studies undertaken by Winstein and others sought to evaluate the properties of metal carbonyl complexes with the homotropylium ion. Comparison between a molybdenum-complex and an iron-complex proved particularly fruitful.
Subsequent NMR studies undertaken by Winstein and others sought to evaluate the properties of metal carbonyl complexes with the homotropylium ion. Comparison between a molybdenum-complex and an iron-complex proved particularly fruitful.



 An intriguing case of Žā-bishomoaromaticity can be found in the dications of pagodanes. In these 4-center-2-electron systems the delocalization happens in the plane that is defined by the four carbon atoms (prototype for the phenomenon of Žā-aromaticity is
An intriguing case of Žā-bishomoaromaticity can be found in the dications of pagodanes. In these 4-center-2-electron systems the delocalization happens in the plane that is defined by the four carbon atoms (prototype for the phenomenon of Žā-aromaticity is 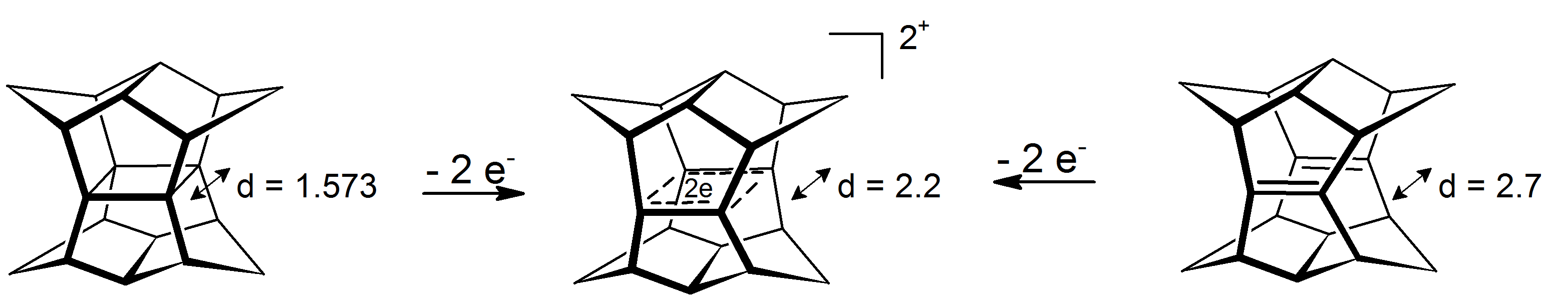 Reduction of the corresponding six electrons dianions was not possible so far.
Reduction of the corresponding six electrons dianions was not possible so far.

 Anionic homoaromaticity can also be seen in dianionic bis-diazene compounds, which contain a four-atom (four nitrogens), six-electron center. Experiment results have shown the shortening of the transannular nitrogen-nitrogen distance, therefore demonstrating that dianionic bis-diazene is a type of anionic bishomoaromatic compound. Peculiar feature of these systems is that the cyclic electron delocalization is taking place in the Žā-plane defined by the four nitrogens. These bis-diazene-dianions are therefore the first examples for 4-center-6-electron Žā-bishomoaromaticity. The corresponding 2 electron Žā-bishomoaromatic systems were realized in the form of pagodane dications (see above).
:
Anionic homoaromaticity can also be seen in dianionic bis-diazene compounds, which contain a four-atom (four nitrogens), six-electron center. Experiment results have shown the shortening of the transannular nitrogen-nitrogen distance, therefore demonstrating that dianionic bis-diazene is a type of anionic bishomoaromatic compound. Peculiar feature of these systems is that the cyclic electron delocalization is taking place in the Žā-plane defined by the four nitrogens. These bis-diazene-dianions are therefore the first examples for 4-center-6-electron Žā-bishomoaromaticity. The corresponding 2 electron Žā-bishomoaromatic systems were realized in the form of pagodane dications (see above).
:
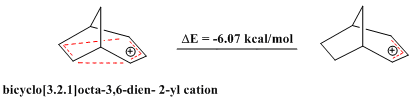 Similarly, a substituted bicyclo .2.1cta-3,6-dien-2-yl cation (the 2-(4'-Fluorophenyl) bicyclo .2.1ct-3,6-dien-2-yl cation) was also shown to be an antiaromate when compared to its corresponding allyl cation, corroborated by theoretical calculations as well as by NMR analysis.
:
Similarly, a substituted bicyclo .2.1cta-3,6-dien-2-yl cation (the 2-(4'-Fluorophenyl) bicyclo .2.1ct-3,6-dien-2-yl cation) was also shown to be an antiaromate when compared to its corresponding allyl cation, corroborated by theoretical calculations as well as by NMR analysis.
:
Homoaromaticity
on the ''
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, refers to a special case of aromaticity
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The e ...
in which conjugation
Conjugation or conjugate may refer to:
Linguistics
*Grammatical conjugation, the modification of a verb from its basic form
*Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
*Complex conjugation, the change o ...
is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbital
In quantum mechanics, an atomic orbital () is a function describing the location and wave-like behavior of an electron in an atom. This function describes an electron's charge distribution around the atom's nucleus, and can be used to calc ...
s, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of ŽĆ electrons that is responsible for this preserved chemical stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system, in particular a chemical compound or a polymer. Colloquially, it may instead refer to kinetic persistence, the shelf-life of a metastable substance or system; t ...
.
Saul Winstein
Saul Winstein (October 8, 1912 – November 23, 1969) was a Jewish Canadian chemist who discovered the ''Winstein reaction.'' He argued a non-classical cation was needed to explain the stability of the norbornyl cation. This fueled a debate ...
in 1959, prompted by his studies of the ŌĆ£tris-homocyclopropenylŌĆØ cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional class of aromatic molecules included under the continuously broadening definition of aromaticity.
To date, homoaromatic compounds are known to exist as cationic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
and anionic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.
Overview
Naming
The term "homoaromaticity" derives from the structural similarity between homoaromatic compounds and the analogous homo-conjugatedalkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbonŌĆōcarbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, ╬▒-olefins.
The Internationa ...
s previously observed in the literature. The IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
Gold Book
The International Union of Pure and Applied Chemistry (IUPAC) publishes many books which contain its complete list of definitions. The definitions are divided initially into seven IUPAC Colour Books: Gold, Green, Blue, Purple, Orange, White, and R ...
requires that Bis-, Tris-, etc. prefixes be used to describe homoaromatic compounds in which two, three, etc. sp3 centers separately interrupt conjugation of the aromatic system.
:
History
The concept of homoaromaticity has its origins in the debate over the non-classicalcarbonium ion
In chemistry, a carbonium ion is a cation that has a pentacoordinated carbon atom. They are a type of carbocation. In older literature, the name "carbonium ion" was used for what is today called carbenium. Carbonium ions charge is delocalized ...
s that occurred in the 1950s. Saul Winstein
Saul Winstein (October 8, 1912 – November 23, 1969) was a Jewish Canadian chemist who discovered the ''Winstein reaction.'' He argued a non-classical cation was needed to explain the stability of the norbornyl cation. This fueled a debate ...
, a famous proponent of the non-classical ion model, first described homoaromaticity while studying the 3-bicyclo .1.0exyl cation.
:solvolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affor ...
reaction occurred empirically faster when the tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
was in the equatorial position. The group ascribed this difference in reaction rates to the anchimeric assistance invoked by the "cis" isomer. This result thus supported a non-classical structure for the cation.
Winstein subsequently observed that this non-classical model of the 3-bicyclo .1.0exyl cation is analogous to the previously well-studied aromatic cyclopropenyl cation. Like the cyclopropenyl cation, positive charge is delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
over three equivalent carbons containing two ŽĆ electrons. This electronic configuration thus satisfies Huckel's rule (requiring 4n+2 ŽĆ electrons) for aromaticity. Indeed, Winstein noticed that the only fundamental difference between this aromatic propenyl cation and his non-classical hexyl cation was the fact that, in the latter ion, conjugation
Conjugation or conjugate may refer to:
Linguistics
*Grammatical conjugation, the modification of a verb from its basic form
*Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
*Complex conjugation, the change o ...
is interrupted by three units. The group thus proposed the name "tris-homocyclopropenyl"ŌĆöthe tris-homo counterpart to the cyclopropenyl cation.
Evidence for homoaromaticity
Criterion for homoaromaticity
The criterion foraromaticity
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The e ...
has evolved as new developments and insights continue to contribute to our understanding of these remarkably stable organic molecule
Some chemical authorities define an organic compound as a chemical compound that contains a carbonŌĆōhydrogen or carbonŌĆōcarbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-cont ...
s. The required characteristics of these molecules has thus remained the subject of some controversy. Classically, aromatic compounds were defined as planar molecules that possess a cyclically delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
system of (4n+2)ŽĆ electrons, satisfying Huckel's rule. Most importantly, these conjugated ring systems are known to exhibit enormous thermochemical stability relative to predictions based on localized resonance structures. Three important features seem to characterize aromatic compounds:
#molecular structure (i.e. coplanarity
In geometry, a set of points in space are coplanar if there exists a geometric plane that contains them all. For example, three points are always coplanar, and if the points are distinct and non-collinear, the plane they determine is unique. How ...
: all contributing atoms in the same plane)
#molecular energetics (i.e. increased thermodynamic stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system, in particular a chemical compound or a polymer. Colloquially, it may instead refer to kinetic persistence, the shelf-life of a metastable substance or system; th ...
)
#spectroscopic
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectrosc ...
and magnetic
Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, m ...
properties (i.e. magnetic field induced ring current)
A number of exceptions to these conventional rules exist, however. Many molecules, including M├Čbius 4nŽĆ electron species, pericyclic transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
s, molecules in which delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
s circulate in the ring plane or through Žā (rather than ŽĆ) bonds, many transition-metal sandwich molecules, and others have been deemed aromatic though they somehow deviate from the conventional parameters for aromaticity.
Consequently, the criterion for homoaromatic delocalization remains similarly ambiguous and somewhat controversial. The homotropylium cation, (C8H9+), though not the first example of a homoaromatic compound ever discovered, has proven to be the most studied of the compounds classified as homoaromatic, and is therefore often considered the classic example of homoaromaticity. By the mid-1980s, there were more than 40 reported substituted derivatives of the homotropylium cation, reflecting the importance of this ion in formulating our understanding of homoaromatic compounds.
Early evidence for homoaromaticity
After initial reports of a "homoaromatic" structure for the tris-homocyclopropenyl cation were published by Winstein, many groups began to report observations of similar compounds. One of the best studied of these molecules is the homotropylium cation, the parent compound of which was first isolated as a stable salt by Pettit, et al. in 1962, when the group reacted cyclooctatraene with strong acids. Much of the early evidence for homoaromaticity comes from observations of unusual NMR properties associated with this molecule.NMR spectroscopy studies
While characterizing the compound resulting from deprotonation of cyclooctatriene by 1HNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic f ...
, the group observed that the resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
corresponding to two proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s bonded to the same methylene bridge carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalentŌĆömeaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
exhibited an astonishing degree of separation in chemical shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of ...
.
: From this observation, Pettit, et al. concluded that the classical structure of the cyclooctatrienyl cation must be incorrect. Instead, the group proposed the structure of the bicyclo .1.0ctadienyl compound, theorizing that the
From this observation, Pettit, et al. concluded that the classical structure of the cyclooctatrienyl cation must be incorrect. Instead, the group proposed the structure of the bicyclo .1.0ctadienyl compound, theorizing that the cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
bond located on the interior of the eight-membered ring must be subject to considerable delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
, thus explaining the dramatic difference in observed chemical shift. Upon further consideration, Pettit was inclined to represent the compound as the "homotropylium ion," which shows the "internal cyclopropane" bond totally replaced by electron delocalization. This structure shows how delocalization is cyclic and involves 6 ŽĆ electrons, consistent with Huckel's rule for aromaticity. The magnetic field of the NMR could thus induce a ring current in the ion, responsible for the significant differences in resonance between the exo and endo protons of this methylene bridge. Pettit, et al. thus emphasized the remarkable similarity between this compound and the aromatic tropylium ion, describing a new "homo-counterpart" to an aromatic species already known, precisely as predicted by Winstein.
: Subsequent NMR studies undertaken by Winstein and others sought to evaluate the properties of metal carbonyl complexes with the homotropylium ion. Comparison between a molybdenum-complex and an iron-complex proved particularly fruitful.
Subsequent NMR studies undertaken by Winstein and others sought to evaluate the properties of metal carbonyl complexes with the homotropylium ion. Comparison between a molybdenum-complex and an iron-complex proved particularly fruitful. Molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
tricarbonyl was expected to coordinate to the homotropylium cation by accepting 6 ŽĆ electrons, thereby preserving the homoaromatic features of the complex. By contrast, iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
tricarbonyl was expected to coordinate to the cation by accepting only 4 ŽĆ electrons from the homotropylium ion, creating a complex in which the electrons of the cation are localized. Studies of these complexes by 1H NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic f ...
showed a large difference in chemical shift values for methylene protons of the Mo-complex, consistent with a homoaromatic structure, but detected virtually no comparable difference in resonance for the same protons in the Fe-complex.
UV spectroscopy studies
An important piece of early evidence in support of the homotropylium cation structure that did not rely on the magnetic properties of the molecule involved the acquisition of its UV spectrum. Winstein et al. determined that the absorption maxima for the homotropylium cation exhibited a considerably shorterwavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
than would be precited for the classical cyclooctatrienyl cation or the bicyclo .1.0ctadienyl compound with the fully formed internal cyclopropane bond (and a localized electronic structure). Instead, the UV spectrum most resembled that of the aromatic tropylium ion. Further calculations allowed Winstein to determine that the bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
between the two carbon atoms adjacent to the outlying methylene bridge is comparable to that of the ŽĆ-bond separating the corresponding carbon atoms in the tropylium cation. Although this experiment proved to be highly illuminating, UV spectra are generally considered to be poor indicators of aromaticity or homoaromaticity.
More recent evidence for homoaromaticity
More recently, work has been done to investigate the structure of the purportedly homoaromatic homotropylium ion by employing various other experimental techniques and theoretical calculations. One key experimental study involved analysis of a substituted homotropylium ion byX-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
. These crystallographic studies have been used to demonstrate that the internuclear distance between the atoms at the base of the cyclopropenyl structure is indeed longer than would be expected for a normal cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
molecule, while the external bonds appear to be shorter, indicating involvement of the internal cyclopropane bond in charge delocalization.
Molecular orbital description
The molecular orbital explanation of the stability of homoaromaticity has been widely discussed with numerous diverse theories, mostly focused on the homotropenylium cation as a reference. R.C. Haddon initially proposed a Mobius model where the outer electrons of the sp3 hybridized methylene bridge carbon(2) back-donate to the adjacent carbons to stabilize the C1-C3 distance.Perturbation molecular orbital theory
Homoaromaticity can better be explained using Perturbation Molecular Orbital Theory (PMO) as described in a 1975 study by Robert C. Haddon. The homotropenylium cation can be considered as a perturbed version of the tropenylium cation due to the addition of a homoconjugate linkage interfering with the resonance of the original cation. :
First-order effects
The most important factor in influencing homoaromatic character is the addition of a single homoconjugate linkage into the parent aromatic compound. The location of the homoconjugate bond is not important as all homoaromatic species can be derived from aromatic compounds that possess symmetry and equal bond order between all carbons. The insertion of a homoconjugate linkage perturbs the ŽĆ-electron density an amount ╬┤╬▓, which depending on the ring size, must be greater than 0 and less than 1, where 0 represents no perturbation and 1 represents total loss of aromaticity (destabilization equivalent to the open chain form). It is believed that with increasing ring size, the resonance stabilization of homoaromaticity is offset by the strain in forming the homoconjugate bridge. In fact, the maximum ring size for homoaromaticity is fairly low as a 16-memberedannulene
Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds (' mancude'). They have the general formula C''n''H''n'' (when ''n'' is an even number) or C''n''H''n''+1 (when ''n'' is an odd num ...
ring favours the formation of the aromatic dication over the strained bridged homocation.
Second-order effects
=Second homoconjugate linkage
= A significant second-order effect on the Perturbation Molecular Orbital model of homoaromaticity is the addition of a second homoconjugate linkage and its influence on stability. The effect is often a doubling of the instability brought about by the addition of a single homoconjugate linkage, although there is an additional term that depends on the proximity of the two linkages. In order to minimize ╬┤╬▓ and thus keep the coupling term to a minimum, bishomoaromatic compounds form depending on the conformation of greatest stability by resonance and smallest steric hindrance. The synthesis of the 1,3-bishomotropenylium cation by protonating cis-bicyclo .1.0ona-2,4,6-triene agrees with theoretical calculations and maximizes stability by forming the two methylene bridges at the 1st and 3rd carbons. :=Substituents
= The addition of a substituent to a homoaromatic compound has a large influence over the stability of the compound. Depending on the relative locations of the substituent and the homoconjugate linkage, the substituent can either have a stabilizing or destabilizing effect. This interaction is best demonstrated by looking at a substituted tropenylium cation. If an inductively electron-donating group is attached to the cation at the 1st or 3rd carbon position, it has a stabilizing effect, improving the homoaromatic character of the compound. However, if this same substituent is attached at the 2nd or 4th carbon, the interaction between the substituent at the homoconjugate bridge has a destabilizing effect. Therefore, protonation of methyl or phenyl substituted cyclooctatetraenes will result in the 1 isomer of the homotropenylium cation. :
Examples of homoaromatic compounds
Following the discovery of the first homoaromatic compounds, research has gone into synthesizing new homoaromatic compounds that possess similar stability to their aromatic parent compounds. There are several classes of homoaromatic compounds, each of which have been predicted theoretically and proven experimentally.Cationic homoaromatics
The most established and well-known homoaromatic species are cationic homoaromatic compounds. As stated earlier, the homotropenylium cation is one of the most studied homoaromatic compounds. Many homoaromatic cationic compounds use as a basis a cyclopropenyl cation, a tropylium cation, or a cyclobutadiene dication as these compounds exhibit strong aromatic character. : In addition to the homotropylium cation, another well established cationic homoaromatic compound is the norbornen-7-yl cation, which has been shown to be strongly homoaromatic, proven both theoretically and experimentally. :cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
which gains about 11.3 kcal molŌłÆ1 stability from the effect). The dications are accessible either via oxidation of pagodane or via oxidation of the corresponding bis-seco-dodecahedradiene:
: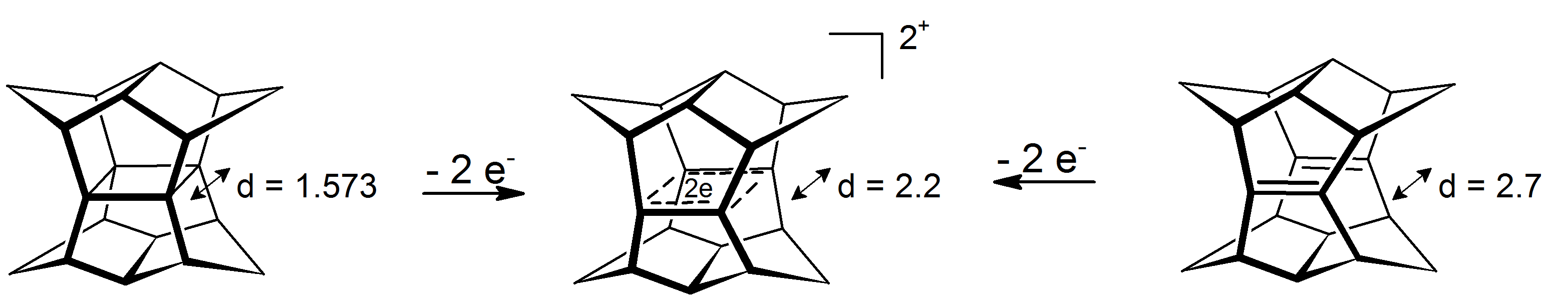 Reduction of the corresponding six electrons dianions was not possible so far.
Reduction of the corresponding six electrons dianions was not possible so far.
Neutral homoaromatics
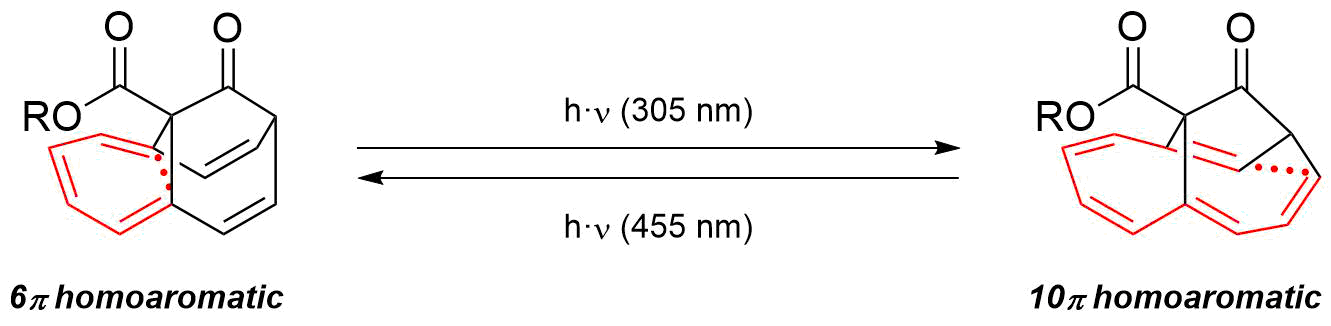
There are many classes of neutral homoaromatic compounds although there is much debate as to whether they truly exhibit homoaromatic character or not. One class of neutral homoaromatics are called monohomoaromatics, one of which is cycloheptatriene, and numerous complex monohomoaromatics have been synthesized. One particular example is a 60-carbon fulleroid derivative that has a single methylene bridge. UV and NMR analysis have shown that the aromatic character of this modified fulleroid is not disrupted by the addition of a homoconjugate linkage, therefore this compound is definitively homoaromatic. Substituted neutral barbaralane derivatives (homoannulenes) have been disclosed as stable ground state homoaromatic molecules in 2023. Evidence for the homoaromatic character in this class of molecules stems from bond length analysis ( X-Ray structural analysis) as well as shifts in theNMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
spectrum. The homoannulenes also act as photoswitches by which means a local 6ŽĆ homoaromaticity can be switched to a global 10ŽĆ homoaromaticity.

Bishomoaromatics
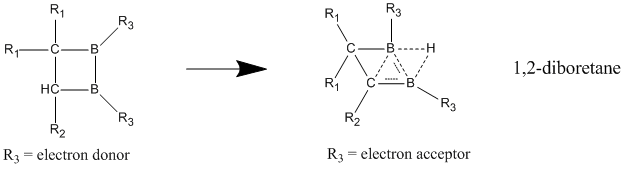
It was long considered that the best examples of neutral homoaromatics are bishomoaromatics such as barrelene and semibullvalene. First synthesized in 1966, semibullvalene has a structure that should lend itself well to homoaromaticity although there has been much debate whether semibullvalene derivatives can provide a true delocalized, ground state neutral homoaromatic compound or not. In an effort to further stabilize the delocalized transition structure by substituting semibullvalene with electron donating and accepting groups, it has been found that the activation barrier to this rearrangement can be lowered, but not eliminated. However, with the introduction of ring strain into the molecule, aimed at destabilizing the localized ground state structure's through the strategic addition of cyclic annulations, a delocalized homoaromatic ground-state structure can indeed be achieved. : Of the neutral homoaromatics, the compounds best believed to exhibit neutral homoaromaticity are boron containing compounds of 1,2-diboretane and its derivatives. Substituted diboretanes are shown to have a much greater stabilization in the delocalized state over the localized one, giving strong indications of homoaromaticity. When electron-donating groups are attached to the two boron atoms, the compound favors a classical model with localized bonds. Homoaromatic character is best seen when electron-withdrawing groups are bonded to the boron atoms, causing the compound to adopt a nonclassical, delocalized structure. :
Trishomoaromatics
As the name suggests, trishomoaromatics are defined as containing one additional methylene bridge compared to bishomoaromatics, therefore containing three of these homoconjugate bridges in total. Just like semibullvalene, there is still much debate as to the extent of the homoaromatic character of trishomoaromatics. While theoretically they are homoaromatic, these compounds show a stabilization of no more than 5% of benzene due to delocalization. :Anionic homoaromatics
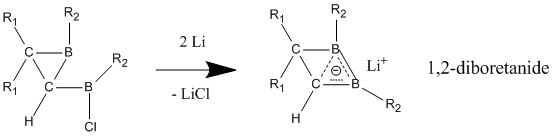
Unlike neutral homoaromatic compounds, anionic homoaromatics are widely accepted to exhibit "true" homoaromaticity. These anionic compounds are often prepared from their neutral parent compounds through lithium metal reduction. 1,2-diboretanide derivatives show strong homoaromatic character through their three-atom (boron, boron, carbon), two-electron bond, which contains shorter C-B bonds than in the neutral classical analogue. These 1,2-diboretanides can be expanded to larger ring sizes with different substituents and all contain some degree of homoaromaticity. : Anionic homoaromaticity can also be seen in dianionic bis-diazene compounds, which contain a four-atom (four nitrogens), six-electron center. Experiment results have shown the shortening of the transannular nitrogen-nitrogen distance, therefore demonstrating that dianionic bis-diazene is a type of anionic bishomoaromatic compound. Peculiar feature of these systems is that the cyclic electron delocalization is taking place in the Žā-plane defined by the four nitrogens. These bis-diazene-dianions are therefore the first examples for 4-center-6-electron Žā-bishomoaromaticity. The corresponding 2 electron Žā-bishomoaromatic systems were realized in the form of pagodane dications (see above).
:
Anionic homoaromaticity can also be seen in dianionic bis-diazene compounds, which contain a four-atom (four nitrogens), six-electron center. Experiment results have shown the shortening of the transannular nitrogen-nitrogen distance, therefore demonstrating that dianionic bis-diazene is a type of anionic bishomoaromatic compound. Peculiar feature of these systems is that the cyclic electron delocalization is taking place in the Žā-plane defined by the four nitrogens. These bis-diazene-dianions are therefore the first examples for 4-center-6-electron Žā-bishomoaromaticity. The corresponding 2 electron Žā-bishomoaromatic systems were realized in the form of pagodane dications (see above).
:
Antihomoaromaticity
There are also reports of antihomoaromatic compounds. Just asaromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
compounds exhibit exceptional stability, antiaromatic compounds, which deviate from Huckel's rule and contain a closed loop of 4n ŽĆ electrons, are relatively unstable. The bridged bicyclo .2.1cta-3,6-dien-2-yl cation contains only 4 ŽĆ electrons, and is therefore "bishomoantiaromatic." A series of theoretical calculations confirm that it is indeed less stable than the corresponding allyl cation.
: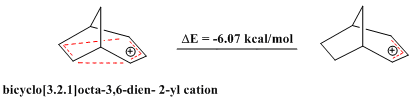 Similarly, a substituted bicyclo .2.1cta-3,6-dien-2-yl cation (the 2-(4'-Fluorophenyl) bicyclo .2.1ct-3,6-dien-2-yl cation) was also shown to be an antiaromate when compared to its corresponding allyl cation, corroborated by theoretical calculations as well as by NMR analysis.
:
Similarly, a substituted bicyclo .2.1cta-3,6-dien-2-yl cation (the 2-(4'-Fluorophenyl) bicyclo .2.1ct-3,6-dien-2-yl cation) was also shown to be an antiaromate when compared to its corresponding allyl cation, corroborated by theoretical calculations as well as by NMR analysis.
:
External links
Homoaromaticity
on the ''
Gold Book
The International Union of Pure and Applied Chemistry (IUPAC) publishes many books which contain its complete list of definitions. The definitions are divided initially into seven IUPAC Colour Books: Gold, Green, Blue, Purple, Orange, White, and R ...
''
References
{{Organic chemistry Physical organic chemistry