Hemiaminal Ether on:
[Wikipedia]
[Google]
[Amazon]
In
 The
The 3 RCHO + 3 NH3 -> (RCHNH)3 + 3 H2O
'' -trisubstituted hexahydro-1,3,5-triazines arise from the condensation of the 3 CH2O + 3 H2NMe -> (CH2NMe)3 + 3 H2O
Although adducts generated from primary amines or ammonia are usually unstable, the hemiaminals have been trapped in a cavity.
Me2NH + CH2O -> Me2NCH2OH
:Me2NH + Me2NCH2OH -> Me2NCH2NMe2 + H2O
The reaction of  Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Methanolamine.svg, methanolamine, an intermediate in the reaction of ammonia with formaldehyde
OC(NHCH2OH)2.png,
 In this reaction step the
In this reaction step the
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, a hemiaminal (also carbinolamine) is a functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
or type of chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
that has a hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
and an amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
attached to the same carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atom: . R can be hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
or an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
group. Hemiaminals are intermediates in imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
formation from an amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
and a carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
by alkylimino-de-oxo-bisubstitution
In organic chemistry, alkylimino-de-oxo-bisubstitution is the organic reaction of carbonyl compounds with amines to imines. The reaction name is based on the IUPAC Nomenclature for Transformations. The reaction is acid catalyzed and the reactio ...
. Hemiaminals can be viewed as a blend of aminal
In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: . (As is customary in organic chemistry, R can represent hydrogen or an alkyl group). A ...
s and geminal diol
A geminal diol (or gem-diol for short) is any organic compound having two hydroxyl functional groups () bound to the same carbon atom. Geminal diols are a subclass of the diols, which in turn are a special class of alcohols. Most of the geminal ...
. They are a special case of amino alcohol
In organic chemistry, alkanolamines (amino alcohols) are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. Alkanolamine's bifunctionality and physicochemical characteristics lead to its u ...
s.
Classification according to amine precursor
Hemiaminals form from the reaction of an amine and a ketone or aldehyde. The hemiaminal is sometimes isolable, but often they spontaneously dehydrate to give imines.Addition of ammonia
 The
The adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
s formed by the addition of ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
to aldehydes have long been studied. Compounds containing both a primary amino
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
group and a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group bonded to the same carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atom are rarely stable ("The hemiaminal erived from primary aminesis, except in very special cases... not observed"), as they tend to dehydrate to form imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s which polymerise to hexamethylenetetramine
Hexamethylenetetramine (HMTA), also known as 1,3,5,7-tetraazaadamantane, is a heterocyclic organic compound with diverse applications. It has the chemical formula (CH2)6N4 and is a white crystalline compound that is highly soluble in water and p ...
. A rare stable example is the adduct of ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It is a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a m ...
, .
The C-substituted derivatives are obtained by reaction of aldehydes and ammonia:
:Addition of primary amines
N-substituted derivatives are somewhat stable. They are invoked but rarely observed as intermediates in theMannich reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia () ...
. These N,N',Namine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
and formaldehyde as illustrated by the route to 1,3,5-trimethyl-1,3,5-triazacyclohexane
1,3,5-Trimethyl-1,3,5-triazinane is an organic compound with the formula (CHNCH). It is a colorless liquid that is soluble in many organic solvents. Structurally, it is one of several related hexahydro-1,3,5-triazines, which typically result fr ...
:
:Addition of secondary amines: carbinolamines (hemiaminals) and bisaminomethanes
One of the simplest reactions entails condensation of formaldehyde and dimethylamine. This reaction produces first the carbinolamine (a hemiaminal) andbis(dimethylamino)methane
Bis(dimethylamino)methane is the organic compound with the formula CH3)2Nsub>2CH2. It is classified as an aminal as well as a ditertiary amine, in fact the simplest. It is a colorless liquid that is widely available. It is prepared by the reac ...
():
:formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
with carbazole
Carbazole is an aromatic Heterocyclic compound, heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is ...
, which is weakly basic, proceed similarly:
: Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Hemiaminal ethers
Hemiaminal ethers have the following structure: R‴-C(NR'2)(OR")-R⁗. Theglycosylamine
Glycosylamines are a class of biochemical compounds consisting of a glycosyl group attached to an amino group, -NR2. They are also known as N-glycosides,. as they are a type of glycoside. Glycosyl groups can be derived from carbohydrates. The gl ...
s are examples of cyclic hemiaminal ethers.
Bis(hydroxymethyl)urea
Bis(hydroxymethyl)urea is an organic compound with the formula OC(NHCH2OH)2. This white water-soluble solid is an intermediate in the formation of urea-formaldehyde resins. It forms upon treatment of urea with an excess of formaldehyde
For ...
is a commercially useful hemiaminal
CF3-stabilizedHemiaminal.svg, An unusual example of an isolable, acyclic hemiaminal: the adduct of ammonia and hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It is a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a m ...
Hemiaminal ether aldehyde.png, Hemiaminal ether derived from an aldehyde
Hemiaminal ether ketone.png, Hemiaminal ether derived from a ketone
Bredereck's reagent.svg, Tert-Butoxybis(dimethylamino)methane
Use in total synthesis
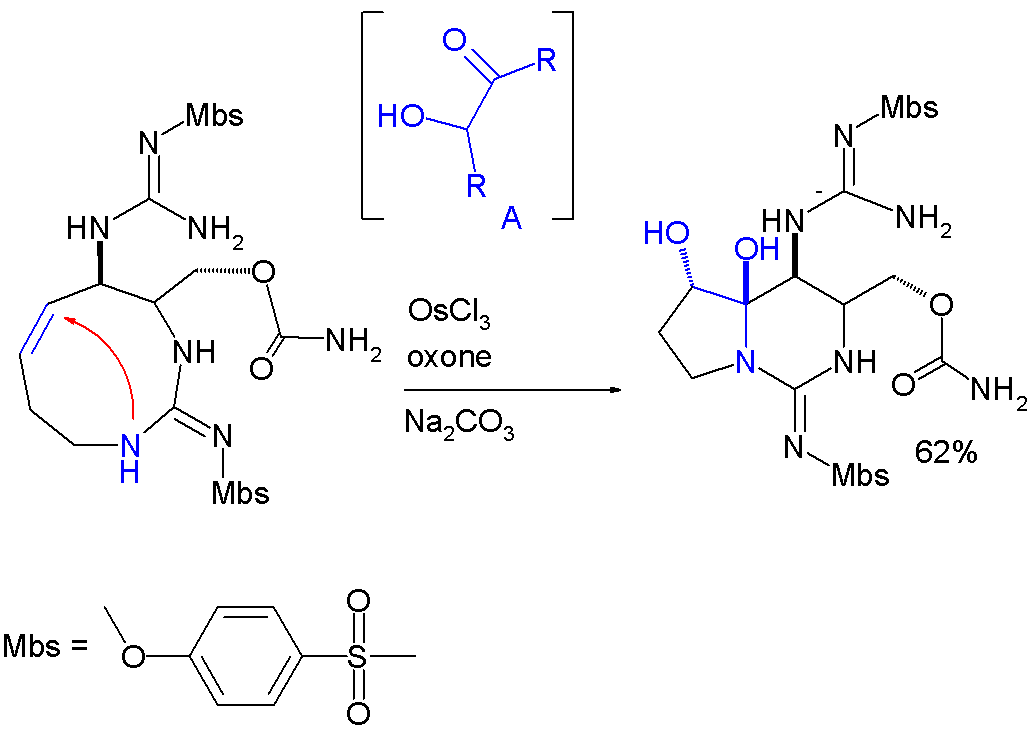
Hemiaminal formation is a key step in an asymmetrictotal synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
of saxitoxin
Saxitoxin (STX) is a potent neurotoxin and the best-known paralytic shellfish toxin. Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic algal blooms, is responsible for the illness known as paralytic she ...
:
: In this reaction step the
In this reaction step the alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
group is first oxidized
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
to an intermediate acyloin
In organic chemistry, acyloins or α-hydroxy ketones are a class of organic compounds of the general form , composed of a hydroxy group () adjacent to a ketone group (). The name ''acyloin'' is derived from the fact that they are formally deri ...
by action of osmium(III) chloride, oxone
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium salt of peroxymonosulfuric acid. Potassium peroxymonosulfate per se is rarely e ...
( sacrificial catalyst) and sodium carbonate
Sodium carbonate (also known as washing soda, soda ash, sal soda, and soda crystals) is the inorganic compound with the formula and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water ...
(base).
See also
*Aminal
In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: . (As is customary in organic chemistry, R can represent hydrogen or an alkyl group). A ...
*Alkanolamine
In organic chemistry, alkanolamines (amino alcohols) are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. Alkanolamine's bifunctionality and physicochemical characteristics lead to its u ...
*Hemiacetal
In organic chemistry, a hemiacetal is a functional group the general formula , where is a hydrogen atom or an organic substituent. They generally result from the nucleophilic Addition reaction, addition of an Alcohol (chemistry), alcohol (a compo ...
References
{{Commonscat, Hemiaminals Functional groups