|
Aminal
In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: . (As is customary in organic chemistry, R can represent hydrogen or an alkyl group). A common aminal is bis(dimethylamino)methane, a colorless liquid that is prepared by the reaction of dimethylamine and formaldehyde: : Aminals are encountered in, for instance, the Fischer indole synthesis. Several examples exist in nature. Physostigmin.svg, Physostigmine, a highly toxic cholinesterase inhibitor found in the Calabar bean. Hodgkinsine.svg, Hodgkinsine, an alkaloid with antiviral, antibacterial and antifungal effects 5,10-methylenetetrahydrofolic acid.svg, 5,10-Methylenetetrahydrofolate, an intermediate in one-carbon metabolism Hexahydro-1,3,5-triazine (), an intermediate in the condensation of formaldehyde and ammonia, tends to degrade to hexamethylene tetraamine. Cyclic aminals can be obtained by the condensation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemiaminal
In organic chemistry, a hemiaminal (also carbinolamine) is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: . R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution. Hemiaminals can be viewed as a blend of aminals and geminal diol. They are a special case of amino alcohols. Classification according to amine precursor Hemiaminals form from the reaction of an amine and a ketone or aldehyde. The hemiaminal is sometimes isolable, but often they spontaneously dehydrate to give imines. Addition of ammonia The adducts formed by the addition of ammonia to aldehydes have long been studied. Compounds containing both a primary amino group and a hydroxyl group bonded to the same carbon atom are rarely stable ("The hemiaminal [derived from primary amines] is, except in very special cases... not observed"), as they tend to de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
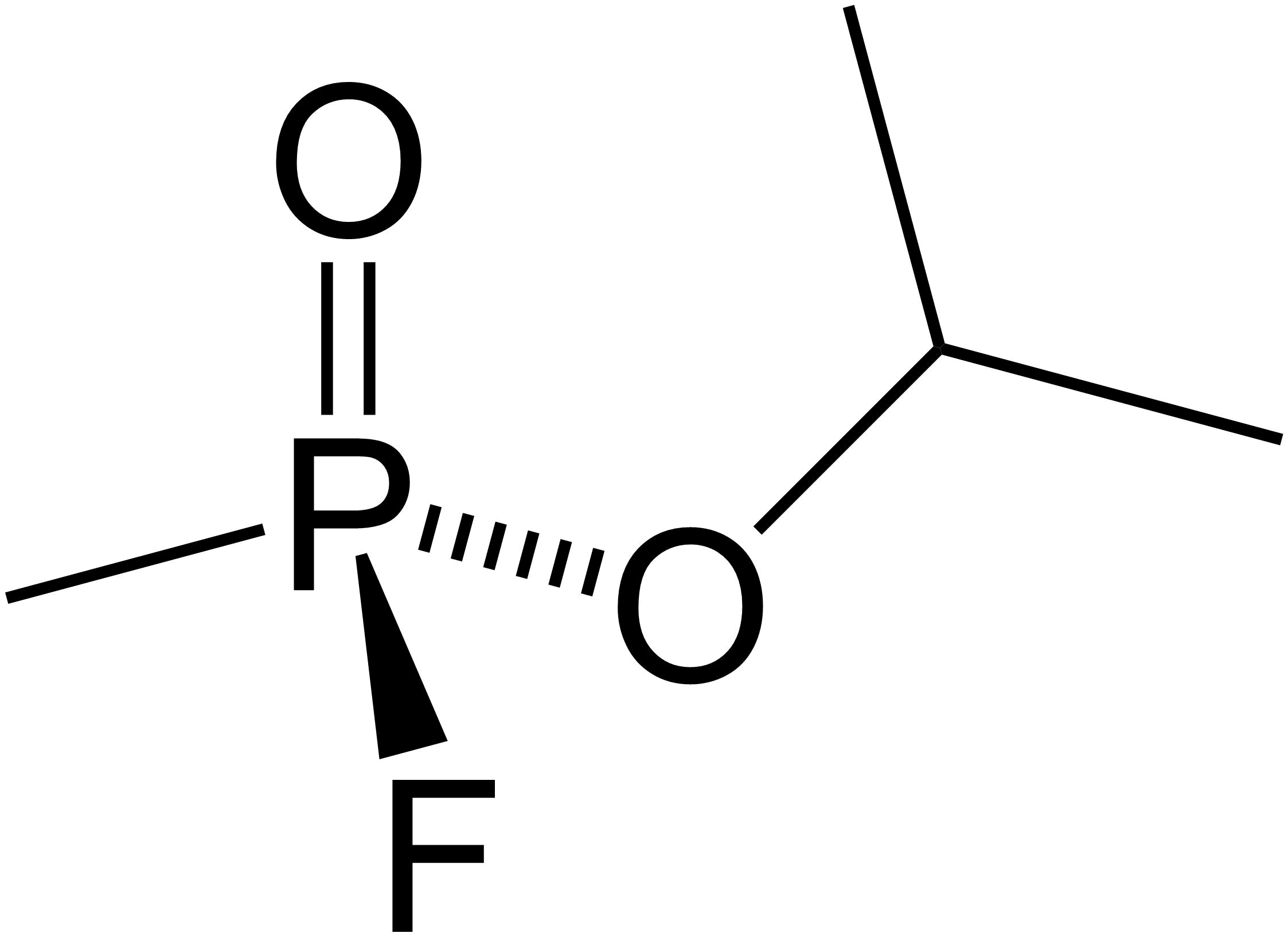
Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry. The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver ( hepatotoxicity). Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypass animal testing, while maintaining the concept of toxicity endpoints. Etymology In Ancient Greek medical literature, the adjective ''τοξικόν'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazolidine
Imidazolidine is a heterocyclic compound (CH2)2(NH)2CH2. The parent imidazolidine is lightly studied, but related compounds substituted on one or both nitrogen centers are more common. Generally, they are colorless, polar, basic compounds. Imidazolidines are cyclic 5-membered examples of the general class of aminals. Preparation Imidazolidines are traditionally prepared by condensation reaction of 1,2-diamines and aldehydes. Most commonly, one or both nitrogen center is substituted with an alkyl or benzyl (Bn) group: : The first unsubstituted imidazolidine synthesis was reported in 1952. Reactions Unsubstituted imidazolidines are often labile. The rings are susceptible to hydrolysis back to the diamine and the aldehyde. Formally, removal of the two hydrogens at carbon 2 (between the two nitrogens) would yield the carbene dihydroimidazol-2-ylidene. Derivatives of the latter comprise an important class of persistent carbenes. Related imidazole-derived heterocycles Classified ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamine
A diamine is an amine with two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to Primary (chemistry), primary diamines, as those are the most reactive. In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine. Vicinal (chemistry), Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry. Aliphatic diamines Linear * 2 carbon backbone: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA. Many 1,2-diamine derivatives are of practical interest such as penicillin. * 3 carbon backbone: 1,3-diaminopropane (propane-1,3-diamine) * 4 carbon backbone: putrescine (butane-1,4-diamine) * 5 carbon backbone: cadaverine (pen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexamethylene Tetraamine
Hexamethylenetetramine (HMTA), also known as 1,3,5,7-tetraazaadamantane, is a heterocyclic organic compound with diverse applications. It has the chemical formula (CH2)6N4 and is a white crystalline compound that is highly soluble in water and polar organic solvents. It is useful in the synthesis of other organic compounds, including plastics, pharmaceuticals, and rubber additives. The compound is also used medically for certain conditions. It sublimes in vacuum at 280°C. It has a tetrahedral cage-like structure similar to adamantane. The four vertices are occupied by nitrogen atoms, which are linked by methylene groups. Although the molecular shape defines a cage, no void space is available at the interior. Synthesis, structure, reactivity Hexamethylenetetramine was discovered by Aleksandr Butlerov in 1859. In this article, Butlerov discovered formaldehyde, which he called "dioxymethylen" (methylene dioxide) age 247because his empirical formula for it was incorrect (C4H4O4). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5,10-Methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer R5,10-methylene-THF. As an intermediate in one-carbon metabolism, 5,10-CH2-THF converts to 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, and methenyltetrahydrofolate. It is substrate for the enzyme methylenetetrahydrofolate reductase (MTHFR), which gives 5-methyltetrahydrofolate. It is mainly produced by the reaction of tetrahydrofolate with serine, catalyzed by the enzyme serine hydroxymethyltransferase. Selected functions Formaldehyde equivalent Methylenetetrahydrofolate is a source of the equivalent of formaldehyde or CH22+ in biosyntheses. Methylenetetrahydrofolate is also an intermediate in the detoxification of formaldehyde. Pyrimidine biosynthesis It is the one-carbon donor for thymidylate synthase, for methylation of 2-deoxy-uridine-5-monophosphate (dUMP) to 2-deoxy-thymidine-5-monophosph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial
An antimicrobial is an agent that kills microorganisms (microbicide) or stops their growth (bacteriostatic agent). Antimicrobial medicines can be grouped according to the microorganisms they are used to treat. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. Antimicrobial medicines to treat infection are known as antimicrobial chemotherapy, while antimicrobial drugs are used to prevent infection, which known as antibiotic prophylaxis, antimicrobial prophylaxis. The main classes of antimicrobial agents are disinfectants (non-selective agents, such as bleach), which kill a wide range of microbes on surfaces to prevent the spread of illness, antiseptics which are applied to living tissue and help reduce infection during surgery, and antibiotics which destroy microorganisms within the body. The term ''antibiotic'' originally described only those formulations derived from living microorga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids. Alkaloids are produced by a large variety of organisms including bacteria, fungus, fungi, Medicinal plant, plants, and animals. They can be purified from crude extracts of these organisms by acid-base extraction, or solvent extractions followed by silica-gel column chromatography. Alkaloids have a wide range of pharmacology, pharmacological activities including antimalarial medication, antimalarial (e.g. quinine), asthma, antiasthma (e.g. ephedrine), chemotherapy, anticancer (e.g. omacetaxine mepesuccinate, homoharringtonine), cholinomimetic (e.g. galantamine), vasodilation, vasodilatory (e.g. vincamine), Antiarrhythmic agent, antiarrhythmic (e.g. quinidine), analgesic (e.g. morphine), antibacterial (e.g. chelerythrine), and anti-diabetic, antihyperglycemic activities (e.g. berb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calabar Bean
''Physostigma venenosum'', the Calabar bean or ordeal bean, is a leguminous plant, Endemism, Endemic to tropical Africa, with a seed poisonous to humans. It derives the first part of its scientific name from a curious beak-like appendage at the end of the carpel, stigma, in the centre of the flower; this appendage, though solid, was supposed to be hollow (hence the name from , a bladder, and stigma). Growth The plant is a large, herbaceous, climbing perennial, with the stem woody at the base, up to in diameter; it has a habit like the scarlet runner, and attains a height of about . The flowers, appearing in axillary peduncles, are large, about long, grouped in pendulous, fascicle (botany), fascicled racemes pale-pink or purplish, and heavily veined. The seed pods, which contain two or three seeds or beans, are in length; and the beans are about the size of an ordinary broad bean, horse bean but less flattened, with a deep chocolate-brown color. Toxicology Calabar bean contains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholinesterase Inhibitor
Cholinesterase inhibitors (ChEIs), also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine by cholinesterase. This increases the amount of the acetylcholine or butyrylcholine in the Chemical synapse#Structure, synaptic cleft that can bind to Muscarinic acetylcholine receptor, muscarinic receptors, Nicotinic acetylcholine receptor, nicotinic receptors and others. This group of inhibitors is divided into two subgroups, acetylcholinesterase inhibitors (AChEIs) and Butyrylcholinesterase#Inhibitors, butyrylcholinesterase inhibitors (BChEIs). ChEIs may be used as drugs for Alzheimer's disease, Alzheimer's and myasthenia gravis, and also as chemical weapons and insecticides. Side effects when used as drugs may include Anorexia (symptom), loss of appetite, nausea, vomiting, Diarrhea#Definition, loose stools, Dream, vivid dreams at night, dehydration, rash, bradycardia, peptic ulcer disease, seizures, weight los ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |