Hell–Volhard–Zelinsky halogenation on:
[Wikipedia]
[Google]
[Amazon]
The Hell–Volhard–Zelinsky halogenation reaction is a chemical transformation that transforms an alkyl 
carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
to the α-bromo derivative. It is a specialized and rare kind of halogenation
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drug ...
.
Examples
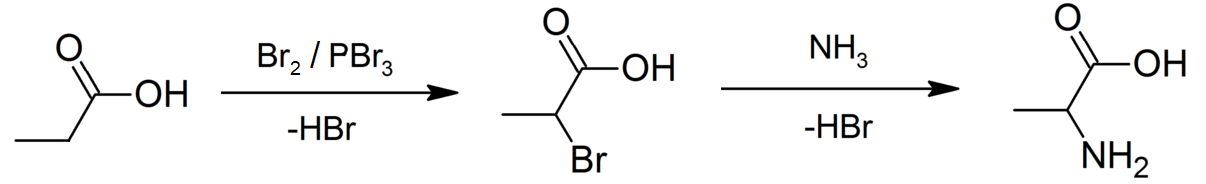
An example of the Hell–Volhard–Zelinsky reaction can be seen in the preparation ofalanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group sid ...
from propionic acid
Propionic acid (, from the Greek language, Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a ...
. In the first step, a combination of bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
and phosphorus tribromide (catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
) is used in the Hell–Volhard–Zelinsky reaction to prepare 2-bromopropionic acid, which in the second step is converted to a racemic mixture
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as r ...
of the amino acid product by ammonolysis
In chemistry, ammonolysis (/am·mo·nol·y·sis/) is the process of splitting ammonia into NH2- + H+. Ammonolysis reactions can be conducted with organic compounds to produce amines (molecules containing a nitrogen atom with a lone pair, :N), o ...
.
:
Mechanism
The reaction is initiated by addition of a catalytic amount of PBr3, after which one molar equivalent of Br2 is added. PBr3 converts the carboxylic OH to the acyl bromide. The acyl bromide tautomerizes to anenol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
, which reacts with the Br2 to brominate at the α position. In neutral to slightly acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
ic aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in wat ...
solution, hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of the α-bromo acyl bromide occurs spontaneously, yielding the α-bromo carboxylic acid. If an aqueous solution is desirable, a full molar equivalent of PBr3 must be used as the catalytic chain is disrupted.
If little nucleophilic solvent is present, reaction of the α-bromo acyl bromide with the carboxylic acid yields the α-bromo carboxylic acid and regenerates the acyl bromide intermediate. In practice a molar equivalent of PBr3 is often used anyway to overcome the slow reaction kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a ...
.
The mechanism for the exchange between an alkanoyl bromide and a carboxylic acid is below.
The α-bromoalkanoyl bromide has a strongly electrophilic carbonyl carbon because of the electron-withdrawing effects of the two bromides.
By quenching the reaction with an alcohol, instead of water, the α-bromo ester can be obtained.
History
The reaction is named after the German chemists Carl Magnus von Hell (1849–1926) and Jacob Volhard (1834–1910) and the Russian chemistNikolay Zelinsky
Nikolay Dmitriyevich Zelinsky (; ; – 31 July 1953) was a Russian and Soviet chemist and educator. He was a professor at Moscow University from 1893 and an academician of the Academy of Sciences of the Soviet Union (1929).
Zelinsky studied at ...
(1861–1953).
See also
* Reformatsky reactionReferences
{{Reflist Halogenation reactions Name reactions