Hectochlorin on:
[Wikipedia]
[Google]
[Amazon]
Hectochlorin is a
 Currently in the MarinLit database, other compounds (besides deacetylhectochlorin and hectochlorin) that contain this fairly unusual gem-dichloro group are lyngbyabellin A-N, 27-deoxylyngbyabellin A and dolabellin, all of those synthesized from Moorena species. Most lyngbyabellins also contain DHIV in their structure, as well as dolabellin. Figure 2 compares the structures of deacetylhectochlorin, hectochlorin, lyngbyabellin B and dolabellin. This figure illustrates the similarities (black) and differences (red) between those compounds compared to deacetylhectochlorin. All of those compounds (but hectochlorin) do not have a proposed biosynthesis published.
Currently in the MarinLit database, other compounds (besides deacetylhectochlorin and hectochlorin) that contain this fairly unusual gem-dichloro group are lyngbyabellin A-N, 27-deoxylyngbyabellin A and dolabellin, all of those synthesized from Moorena species. Most lyngbyabellins also contain DHIV in their structure, as well as dolabellin. Figure 2 compares the structures of deacetylhectochlorin, hectochlorin, lyngbyabellin B and dolabellin. This figure illustrates the similarities (black) and differences (red) between those compounds compared to deacetylhectochlorin. All of those compounds (but hectochlorin) do not have a proposed biosynthesis published.

lipopeptide
A lipopeptide is a molecule consisting of a lipid connected to a peptide. They are able to self-assemble into different structures. Many bacteria produce these molecules as a part of their metabolism, especially those of the genus ''Bacillus'', ...
that exhibits potent antifungal
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as ...
activity against '' C. albicans'' and a number of plants pathogens, as well as inhibiting growth of human cell lines by hyperpolymerization of actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ...
. It was originally isolated from the filamentous cyanobacterium
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteria' ...
'' Moorena producens'' JHB, collected from Hector Bay, Jamaica, 1996, which is a strain also known for being the producer of other two potent biomolecules named Jamaicamide A
Jamaicamide A is a lipopeptide isolated from the cyanobacterium ''Moorea producens'', formerly known as ''Lyngbya majuscula''. Jamaicamide A belongs to a family of compounds collectively called jamaicamides, which are sodium channel blockers with ...
and Cryptomaldamide. Due to its activity against plants pathogens, synthetic efforts elucidated the compound’s total synthesis in 2002. ''Moorena'' species are normally the main component of the dietary of some sea hare
The order Aplysiida, commonly known as sea hares ('' Aplysia'' species and related genera), are medium-sized to very large opisthobranch gastropod molluscs with a soft internal shell made of protein. These are marine gastropod molluscs in t ...
s, which concentrate the cyanobacterial metabolites as a mechanism of defense from predators. Therefore, in 2005, hectochlorin was re-isolated from the Thai sea hare ''Bursatella leachii
''Bursatella leachii'', whose common name is the ragged sea hare or shaggy sea hare, is a species of large sea slug: a marine (ocean), marine gastropod mollusk in the sea hare family Aplysiidae.MolluscaBase eds. (2021). MolluscaBase''Bursatella ...
'', along with a new analogue, deacetylhectochlorin.Suntornchashwej, S., Chaichit, N., Isobe, M. & Suwanborirux, K. Hectochlorin and morpholine derivatives from the Thai sea hare, Bursatella leachii. J. Nat. Prod. 68, 951–955 (2005). Another reisolation of hectochlorin was reported in 2013, from another ''Moorena producens'' strain (RS05), isolated from the Red Sea
The Red Sea is a sea inlet of the Indian Ocean, lying between Africa and Asia. Its connection to the ocean is in the south, through the Bab-el-Mandeb Strait and the Gulf of Aden. To its north lie the Sinai Peninsula, the Gulf of Aqaba, and th ...
, surprising in a non-tropical environment, as opposed to the other Moorena strains isolated before. The predicted biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthe ...
of hectochlorin was published in 2007 and consists in a hybrid NRPS-PKS, with a hexanoic acid
Caproic acid, also known as hexanoic acid, is the carboxylic acid derived from hexane with the chemical formula . It is a colorless oily liquid with a fatty, cheesy, waxy odor resembling that of goats or other barnyard animals. It is a fatty acid ...
as start unit that becomes halogenated twice in the position 5, producing fairly rare gem-dichloro group, that along with two 2,3-dihydroxyisovaleric acid (DHIV) units compose a very interesting bioactive molecule.
Biosynthesis
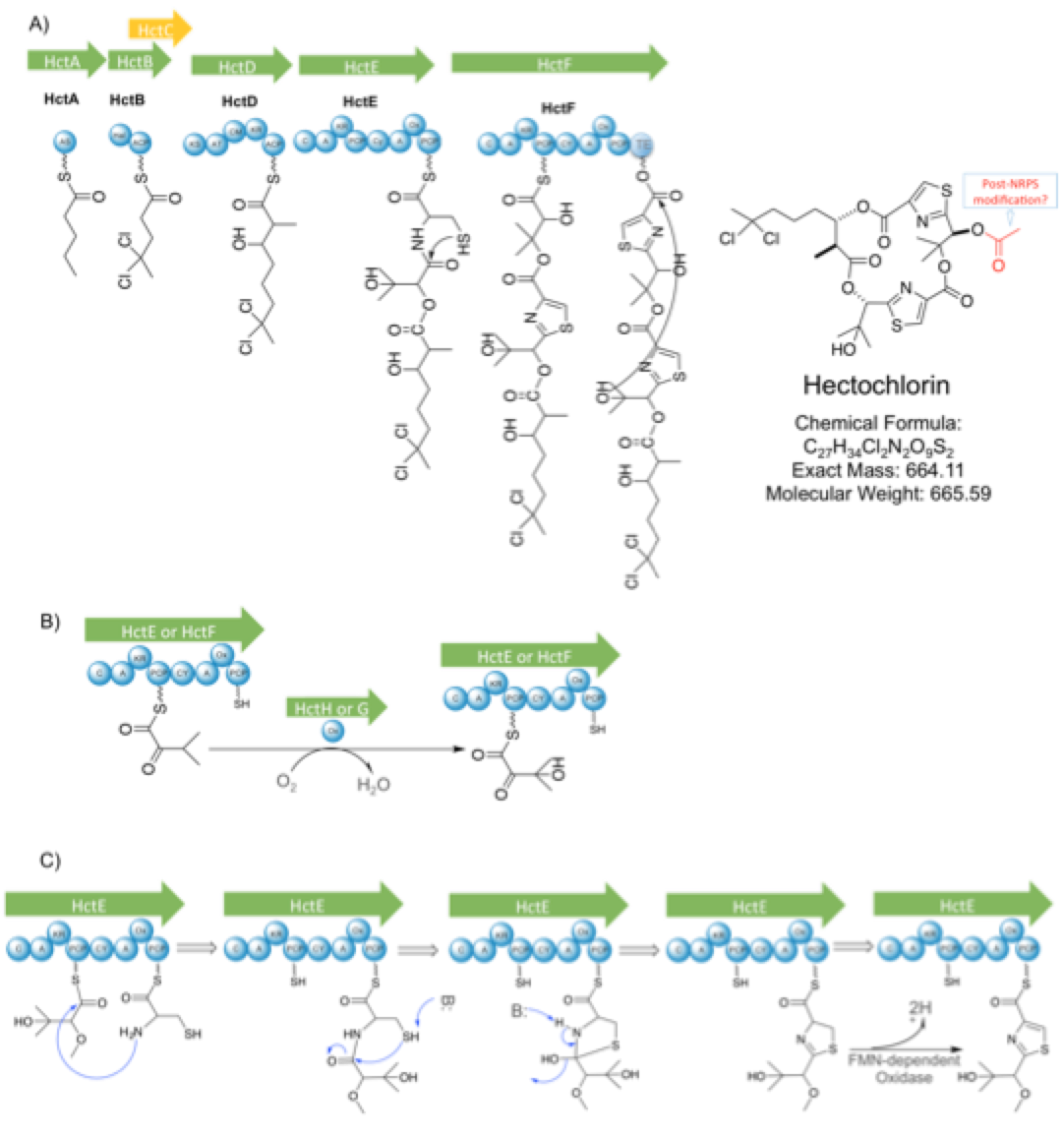
The biosynthetic gene cluster (BGC) is composed of eight genes (Figure 1A), of which seven are directly related to the synthesis of the molecule (hctA-B and hctD-H, in green) and one is predicted to encode atransposase
A transposase is any of a class of enzymes capable of binding to the end of a transposon and catalysing its movement to another part of a genome, typically by a cut-and-paste mechanism or a replicative mechanism, in a process known as transpositio ...
(hctC, in yellow), that tends to be related to the mobility of the gene and not the synthesis of molecule features. The cluster is also flanked by other 5 ORF ( Open reading rrames), including three hypothetical proteins, a homing endonuclease
In molecular biology, endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain (namely DNA or RNA). Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically (with regard to sequence), while man ...
and a reverse transcriptase
A reverse transcriptase (RT) is an enzyme used to convert RNA genome to DNA, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genomes, by retrotransposon mobi ...
of unknown function regarding biosynthesis mechanistic of the molecule.
Biosynthesis of hectochlorin starts with hctA (Figure 1A), responsible for the start unit, which has 53% similarity to an Acyl-ACP synthetase in ''Fischerella
''Fischerella'' is a genus of cyanobacteria belonging to the family Hapalosiphonaceae.
The genus was first described by M. Gomont in 1895.
The genus has cosmopolitan distribution.
The genus name of ''Deightoniella'' is in honour of Christian F ...
'' and is predicted to generate a hexanoic acid
Caproic acid, also known as hexanoic acid, is the carboxylic acid derived from hexane with the chemical formula . It is a colorless oily liquid with a fatty, cheesy, waxy odor resembling that of goats or other barnyard animals. It is a fatty acid ...
that starts the hectochlorin molecule. This hexanoic acid gets halogenated twice at the fifth carbon by the gene hctB, generating the gem-dichloro group in 5,5-dichlorohexanoic acid. For such, hctB has one halogenation domain in the N-terminus, which is 47% similar to a halogenase at ''Microcystis aeruginosa'' and also contains all conserved residues for the binding of Fe2+/2- oxoglutarate co-factor. In the C-terminus, one ACP domain is present and it is fairly homologous to several others ACP domain at cyanobacteria, like Curacin A, Jamaicamide. As mentioned before, hctC is a transposase of unknown function and it is not directly related in the molecule synthesis. Next, this 5,5-dichlorohexanoic acquires one KS extension by hctD. This gene consists of a single KS module with a minimal configuration (KS-AT-CP) plus one KR and cMT, producing a 7,7-dichloro-3-hydroxy-2-methyl-octanoic acid. HctE consists of a bi-modular NRPS, of which the first module incorporates an isovaleric acid
Isovaleric acid, also known as 3-methylbutanoic acid or β-methylbutyric acid, is a branched-chain alkyl carboxylic acid with the chemical formula (CH3)2CHCH2CO2H. It is classified as a short-chain fatty acid. Like other low-molecular-weight car ...
and the second module incorporates a heterocyclic cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
. In the first module, the adenlyation-domain (A domain) has a mutation substituting a conserved aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protein ...
residue (Asp235) that is related to interactions with the amino group of the cognate amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
, therefore, incorporating isovaleric acid instead an amino acid. This hydroxyl group that substitutes an amino group condensates with the previous carbonyl from the KS extension. The second module has 67% identity to CurF and BarG (from Curacin A and Barbamide BGCs) and is predicted to adenylate and heterocyclize a cysteine, as well as oxidize it by FMN-dependent oxidase present in between adenlyation conserved motifs, catalyzing the formation of a thiazole
Thiazole (), or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the ...
ring (Figure 1C). HctF gene has remarkable similarity to hctE, although, there are two main differences: the iso-valeric acid incorporated does not condensate by the hydroxyl group that substitute an amino group, instead, it condensates be the hydroxyl group in the side chain created by a P450 oxidation (Figure 1B); hctF has an extra thioesterase
In biochemistry, thioesterases are enzymes which belong to the esterase family. Esterases, in turn, are one type of the several hydrolases known.
Thioesterases exhibit esterase activity (splitting of an ester into an acid and an alcohol, in the ...
domain that converts thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
bond to ester bond and catalyze the attack from the C-terminal free hydroxyl group to this newly formed ester, cyclizing the molecule. The final genes hctG and hctH probably encode two P450 that oxidize the side chain of both isovaleric acids (Figure 1D). Lastly, a post-NRPS modification happens in the free hydroxyl group from the second isovaleric acid, adding an acetyl group
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl grou ...
. This addition is not predicted in the current biosynthesis, although the absence of this post-NRPS modification would produce the analogue previously mentioned, deacetylhectochlorin.
 Currently in the MarinLit database, other compounds (besides deacetylhectochlorin and hectochlorin) that contain this fairly unusual gem-dichloro group are lyngbyabellin A-N, 27-deoxylyngbyabellin A and dolabellin, all of those synthesized from Moorena species. Most lyngbyabellins also contain DHIV in their structure, as well as dolabellin. Figure 2 compares the structures of deacetylhectochlorin, hectochlorin, lyngbyabellin B and dolabellin. This figure illustrates the similarities (black) and differences (red) between those compounds compared to deacetylhectochlorin. All of those compounds (but hectochlorin) do not have a proposed biosynthesis published.
Currently in the MarinLit database, other compounds (besides deacetylhectochlorin and hectochlorin) that contain this fairly unusual gem-dichloro group are lyngbyabellin A-N, 27-deoxylyngbyabellin A and dolabellin, all of those synthesized from Moorena species. Most lyngbyabellins also contain DHIV in their structure, as well as dolabellin. Figure 2 compares the structures of deacetylhectochlorin, hectochlorin, lyngbyabellin B and dolabellin. This figure illustrates the similarities (black) and differences (red) between those compounds compared to deacetylhectochlorin. All of those compounds (but hectochlorin) do not have a proposed biosynthesis published.

References
{{reflist Thiazoles Lipopeptides Organochlorides Heterocyclic compounds with 3 rings Acetate esters Lactones Tertiary alcohols Halogen-containing natural products