Grob Fragmentation on:
[Wikipedia]
[Google]
[Amazon]
A Grob fragmentation is an  The reaction is named for the Swiss chemist .
Alternately, atom 1 could begin as an anion, in which case it becomes neutral rather than going from neutral to cationic.
The reaction is named for the Swiss chemist .
Alternately, atom 1 could begin as an anion, in which case it becomes neutral rather than going from neutral to cationic.

 The original work by Grob (1955) concerns the formation of 1,5-hexadiene from ''cis''- or ''trans''-1,4-dibromocyclohexane by
The original work by Grob (1955) concerns the formation of 1,5-hexadiene from ''cis''- or ''trans''-1,4-dibromocyclohexane by  According to reviewers Prantz and Mulzer (2010), the name Grob fragmentation was chosen "in more or less glaring disregard of the earlier contributions".
According to reviewers Prantz and Mulzer (2010), the name Grob fragmentation was chosen "in more or less glaring disregard of the earlier contributions".
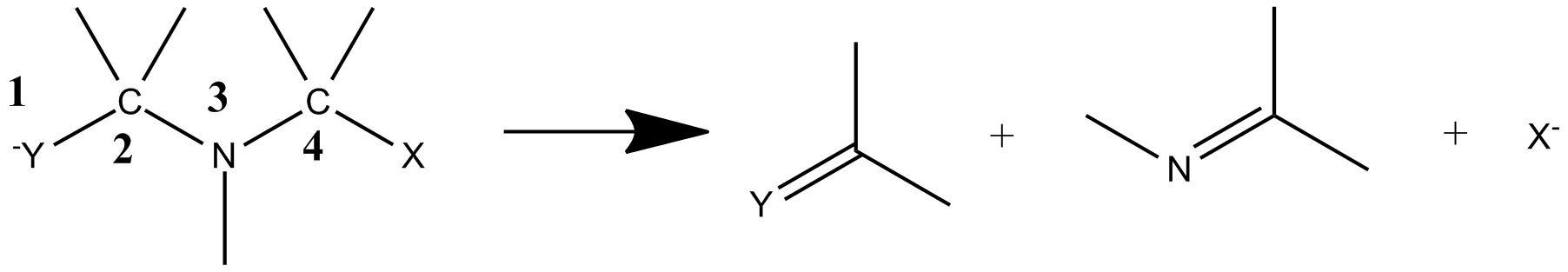 3-aza-Grob fragmentation can proceed with several different nucleofuges. The
3-aza-Grob fragmentation can proceed with several different nucleofuges. The  The scope of the reaction has been found to cover
The scope of the reaction has been found to cover
elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
that breaks a neutral aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
chain into three fragments: a positive ion spanning atoms 1 and 2 (the "electrofuge
In chemistry, an electrofuge is a leaving group that does not retain the lone pair of electrons from its previous bond with another species (in contrast to a nucleofuge, which does). It can result from the heterolytic breaking of covalent bonds. ...
"), an unsaturated neutral fragment spanning positions 3 and 4, and a negative ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
(the "nucleofuge
In chemistry, a nucleofuge () is a leaving group which retains the lone pair of electrons from its previous bond with another species. For example, in the SN2 mechanism, a nucleophile attacks an organic compound containing the nucleofuge (the b ...
") comprising the rest of the chain.
For example, the positive ion may be a carbenium
The carbenium ion is a kind of positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a ...
, carbonium
In chemistry, a carbonium ion is a cation that has a pentacoordinated carbon atom. They are a type of carbocation. In older literature, the name "carbonium ion" was used for what is today called carbenium. Carbonium ions charge is delocalized i ...
or acylium ion In organic chemistry, acylium ions are cations with the formula RCO+, where R = alkyl or aryl. They are a kind of carbocation.
Structure, bonding, synthesis
In acylium ions, the C-C-O linkage is linear. The oxygen and the central carbon can be d ...
; the neutral fragment could be an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
, alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
, or imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
; and the negative fragment could be a tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
or hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
ion:
History
An early instance of fragmentation is thedehydration
In physiology, dehydration is a lack of total body water that disrupts metabolic processes. It occurs when free water loss exceeds intake, often resulting from excessive sweating, health conditions, or inadequate consumption of water. Mild deh ...
of di(''tert''-butyl)methanol yielding 2-methyl-2-butene
2-Methyl-2-butene, 2m2b, 2-methylbut-2-ene, beta-isoamylene, or trimethylethylene is an alkene hydrocarbon with the molecular formula C5H10.
Used as a free radical scavenger in trichloromethane (chloroform) and dichloromethane (methylene chloride ...
and isobutene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Productio ...
, a reaction described in 1933 by Frank C. Whitmore. This reaction proceeds by formation of a secondary carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
followed by a rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another at ...
to a more stable tertiary carbocation and elimination of a ''t''-butyl cation:
Albert Eschenmoser
Albert Jakob Eschenmoser (5 August 1925 – 14 July 2023) was a Swiss organic chemist, best known for his work on the synthesis of complex heterocyclic natural compounds, most notably vitamin B12. In addition to his significant contributions to ...
in 1952 investigated the base catalysed fragmentation of certain beta hydroxy ketones:
sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
metal:
Reaction mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
varies with reactant and reaction conditions with the fragmentation taking place in a concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not ...
or taking place in two steps with a carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
ic intermediate when the nucleofuge leaves first or taking place in two steps with an anionic intermediate when the electrofuge leaves first. The carbanionic pathway is more common and is facilitated by the stability of the cation formed and the leaving group ability of the nucleofuge. With cyclic substrates, the preferred geometry of elimination is for the sigma bond that drives out the leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
to being anti to it, analogous to the conformational orientation in the E2 mechanism of elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
s.
Examples
Thapsigargin from Wieland–Miescher ketone
An example of a Grob-like fragmentation inorganic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
is the expansion of the Wieland–Miescher ketone to thapsigargin
Thapsigargin is a non-competitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase ( SERCA). Structurally, thapsigargin is classified as a guaianolide, and is extracted from a plant, '' Thapsia garganica''. It is a tumor promoter in ...
:
In this reaction, diastereoselective reduction of the ketone 1 with sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
yields alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
2, which is functionalized to the mesylate
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the gr ...
3 with mesyl chloride
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula . Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group –, it is frequently abbreviated MsCl in reaction schemes or equations. It ...
in pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
. The selectivity of the initial reduction of ketone 1 is a result of borohyride approaching from the bottom face to avoid steric clash with the axial methyl group. Then reduction of the enone to allyl alcohol
Allyl alcohol (IUPAC name: prop-2-en-1-ol) is an organic compound with the structural formula . Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to ...
4 with tri-''tert''-butoxyaluminium hydride in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
followed by hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration ...
with borane
Borane is an inorganic compound with the chemical formula . Because it tends to dimerize or form adducts, borane is very rarely observed. It normally dimerizes to diborane in the absence of other chemicals. It can be observed directly as a c ...
in THF yields the borane 5 (only one substituent displayed for clarity). The diastereoselectivity of the hydroboration is a result of two factors: avoidance of the axial methyl group as well as axial hydride addition to avoid a twist-boat conformation in the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
. The Grob fragmentation to 6 takes place with sodium methoxide
Sodium methoxide is the simplest sodium alkoxide. With the formula , it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
...
in methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
at reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to Chemical ...
. A methoxide group attacks the boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
atom giving a borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
complex which fragments. As each boron atom can hold three substrate molecules (R), the ultimate boron byproduct is trimethyl borate
Trimethyl borate is the organoboron compound with the formula B(OCH3)3. It is a colourless liquid that burns with a green flame. It is an intermediate in the preparation of sodium borohydride and is a popular reagent in organic chemistry. It i ...
. As seen in 6, the mesylate being in the equatorial position allows its sigma star orbital to align ideally with the sigma bond drawn, allowing for the correct olefin geometry seen in 7.
Another example is an epoxy alcohol fragmentation reaction as part of the Holton Taxol total synthesis.
aza-Grob fragmentation
3-aza-Grob fragmentation is variation which takes place when anelectrofuge
In chemistry, an electrofuge is a leaving group that does not retain the lone pair of electrons from its previous bond with another species (in contrast to a nucleofuge, which does). It can result from the heterolytic breaking of covalent bonds. ...
and nucleofuge
In chemistry, a nucleofuge () is a leaving group which retains the lone pair of electrons from its previous bond with another species. For example, in the SN2 mechanism, a nucleophile attacks an organic compound containing the nucleofuge (the b ...
are situated at positions 1 and 5 on a secondary or tertiary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
chain with the nitrogen at the 3 position. The reaction products are an electrofugal fragment, an imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
, and a nucleofugal fragment (such as an alcohol).
 3-aza-Grob fragmentation can proceed with several different nucleofuges. The
3-aza-Grob fragmentation can proceed with several different nucleofuges. The reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
has been reported to begin with the reduction of an ether protected amide to form a secondary alcohol. Fragmentation then takes place in a concerted step to form the reaction products.
 The scope of the reaction has been found to cover
The scope of the reaction has been found to cover THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
and tetrahydrothiophene
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene and is therefore the sulf ...
protecting groups using various hydride agents.
See also
* Eschenmoser fragmentation * Wharton reactionReferences
{{Reflist Elimination reactions Name reactions