GroEL on:
[Wikipedia]
[Google]
[Amazon]
GroEL is a protein which belongs to the
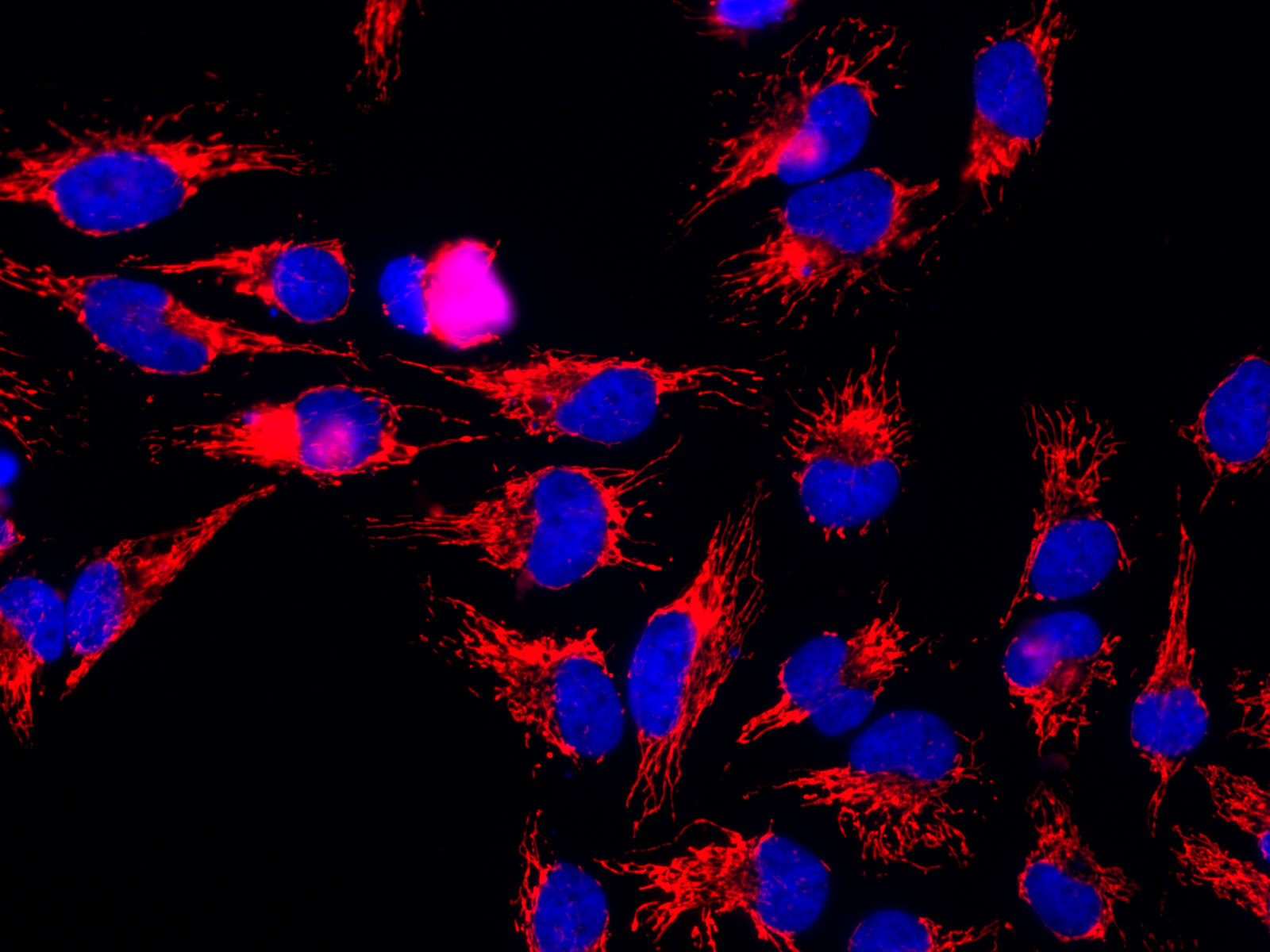 The mitochondrial HSP60
The mitochondrial HSP60
Image:GroEL.png, GroEL (side)
Image:GroEL top.png, GroEL (top)
Image:GroES-GroEL.png, GroES/GroEL complex (side)
Image:GroES-GroEL top.png, GroES/GroEL complex (top)
The key to the activity of GroEL is in the structure of the monomer. The Hsp60 monomer has three distinct sections separated by two hinge regions. The apical section contains many hydrophobic
3D macromolecular structures of GroEL in EMDB
{{Chaperones Moonlighting proteins Protein complexes Molecular chaperones
chaperonin
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 b ...
family of molecular chaperones, and is found in many bacteria. It is required for the proper folding of many proteins. To function properly, GroEL requires the lid-like cochaperonin protein complex GroES
Heat shock 10 kDa protein 1 (Hsp10), also known as chaperonin 10 (cpn10) or early-pregnancy factor (EPF), is a protein that in humans is encoded by the ''HSPE1'' gene. The homolog in ''Escherichia coli, E. coli'' is GroES that is a chaperonin ...
. In eukaryotes
The eukaryotes ( ) constitute the domain of Eukaryota or Eukarya, organisms whose cells have a membrane-bound nucleus. All animals, plants, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of ...
the organellar proteins Hsp60 and Hsp10 are structurally and functionally nearly identical to GroEL and GroES, respectively, due to their endosymbiotic origin.
HSP60 is implicated in mitochondrial protein import and macromolecular assembly. It may facilitate the correct folding of imported proteins, and may also prevent misfolding and promote the refolding and proper assembly of unfolded polypeptides generated under stress conditions in the mitochondrial matrix. HSP60 interacts with HRAS and with HBV protein X and HTLV-1 protein p40tax. HSP60 belongs to the chaperonin (HSP60) family. Note: This description may include information from UniProtKB.
Alternate Names: 60 kDa chaperonin, Chaperonin 60, CPN60, Heat shock protein 60, HSP-60, HuCHA60, Mitochondrial matrix protein P1, P60 lymphocyte protein, HSPD1
Heat shock protein 60 (HSP60) is a mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
l chaperonin
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 b ...
that is typically held responsible for the transportation and refolding of proteins from the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
into the mitochondrial matrix
In the mitochondrion, the matrix is the space within the inner membrane. It can also be referred as the mitochondrial fluid. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitoc ...
. In addition to its role as a heat shock protein, HSP60 functions as a chaperonin to assist in folding linear amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
chains into their respective three-dimensional structure. Through the extensive study of groEL, HSP60’s bacterial
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the ...
homolog, HSP60 has been deemed essential in the synthesis and transportation of essential mitochondrial proteins from the cell's cytoplasm into the mitochondrial matrix. Further studies have linked HSP60 to diabetes
Diabetes mellitus, commonly known as diabetes, is a group of common endocrine diseases characterized by sustained high blood sugar levels. Diabetes is due to either the pancreas not producing enough of the hormone insulin, or the cells of th ...
, stress response, cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
and certain types of immunological
Immunology is a branch of biology and medicine that covers the study of immune systems in all organisms.
Immunology charts, measures, and contextualizes the physiological functioning of the immune system in states of both health and disease ...
disorders.
Discovery
Not much is known about the function of HSP60.Mammal
A mammal () is a vertebrate animal of the Class (biology), class Mammalia (). Mammals are characterised by the presence of milk-producing mammary glands for feeding their young, a broad neocortex region of the brain, fur or hair, and three ...
ian HSP60 was first reported as a mitochondrial P1 protein. It was subsequently cloned and sequenced
In genetics and biochemistry, sequencing means to determine the primary structure (sometimes incorrectly called the primary sequence) of an unbranched biopolymer. Sequencing results in a symbolic linear depiction known as a sequence which succi ...
by Radhey Gupta and coworkers. The amino acid sequence showed a strong homology to GroEL. It was initially believed that HSP60 functioned only in the mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
and that there was no equivalent protein located in the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
. Recent discoveries have discredited this claim and have suggested that there is a recognizable difference between HSP60 in the mitochondria and in the cytoplasm. A similar protein structure exists in the chloroplast
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur ...
of certain plants. This protein presence provides evidence for the evolutionary relationship of the development of the mitochondria and the chloroplast by means of endosymbiosis
An endosymbiont or endobiont is an organism that lives within the body or cells of another organism. Typically the two organisms are in a mutualism (biology), mutualistic relationship. Examples are nitrogen-fixing bacteria (called rhizobia), whi ...
.
Structure
Under normal physiological conditions, HSP60 is a 60 kilodalton oligomer composed of monomers that form a complex arranged as two stacked heptameric rings. This double ring structure forms a large central cavity in which the unfolded protein binds viahydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
interactions. This structure is typically in equilibrium with each of its individual components: monomers, heptamers, and tetradecamers. Recent studies have begun to suggest that in addition to its typical location in the mitochondria, HSP60 can also be found in the cytoplasm under normal physiological conditions.
Each subunit of HSP60 has three domains: the apical domain, the equatorial domain, and the intermediate domain. The equatorial domain contains the binding site for ATP and for the other heptameric ring. The intermediate domain binds the equatorial domain and the apical domain together. The intermediate domain induces a conformational change when ATP is bound allowing for an alternation between the hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
and hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
substrate binding sites. In its inactive state, the protein is in a hydrophobic state. When activated by ATP, the intermediate domain undergoes a conformational change that exposes the hydrophilic region. This insures fidelity in protein binding. Chaperonin 10 aids HSP60 in folding by acting as a dome-like cover on the ATP active form of HSP60. This causes the central cavity to enlarge and aids in protein folding. See the above figure for further detail on the structure.
 The mitochondrial HSP60
The mitochondrial HSP60 sequence
In mathematics, a sequence is an enumerated collection of objects in which repetitions are allowed and order matters. Like a set, it contains members (also called ''elements'', or ''terms''). The number of elements (possibly infinite) is cal ...
contains a series of G repeats at the C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When t ...
. The structure and function of this sequence is not quite known. The N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
contains a pre-sequence of hydroxylated amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
, namely arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidinium, guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) a ...
, lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group ( ...
, serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
, and threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− ...
, which serve as directors for the importation of the protein into the mitochondria.
The predicted structure of HSP60 includes several vertical sine waves
A sine wave, sinusoidal wave, or sinusoid (symbol: ∿) is a periodic wave whose waveform (shape) is the trigonometric sine function. In mechanics, as a linear motion over time, this is '' simple harmonic motion''; as rotation, it correspond ...
, alpha helices
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of l ...
, beta sheets, and 90 degree turns. There are regions of hydrophobicity
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly intermolecular force, repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to b ...
where the protein presumably spans the membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
. There are also three N-linked glycosylation sites at positions 104, 230, 436. The sequence and secondary structure for the mitochondrial protein are illustrated in the above image obtained from the Protein Data Bank.
Newer information has begun to suggest that the HSP60 found in the mitochondria differs from that of the cytoplasm. With respect to the amino acid sequence, the cytoplasmic HSP60 has an N-terminal sequence not found in the mitochondrial protein. In gel electrophoresis
Gel electrophoresis is an electrophoresis method for separation and analysis of biomacromolecules (DNA, RNA, proteins, etc.) and their fragments, based on their size and charge through a gel. It is used in clinical chemistry to separate ...
analysis, significant differences were found in the migration of cytoplasmic and mitochondrial HSP60. The cytoplasmic HSP60 contains a signal sequence of 26 amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
on the N terminus. This sequence is highly degenerate and is capable of folding into amphiphilic helix. Antibodies
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that caus ...
against HSP60 targeted both the mitochondrial and cytoplasmic form. Nonetheless, antibodies against the signal sequence targeted only the cytoplasmic form. Under normal physiological condition, both are found in relatively equal concentrations. In times of stress or high need of HSP60 in either the cytoplasm or the mitochondria, the cell is capable for compensating by increasing the presence of HSP60 in one compartment and decreasing its concentration in the opposite compartment.
Function
Common
Heat shock proteins are amongst the most evolutionarily conserved of proteins. The significant function, structural, and sequential homology between HSP60 and its prokaryotic homolog, groEL, demonstrates this level of conservation. Moreover, HSP60’s amino acid sequence bears a similarity to its homolog inplants
Plants are the eukaryotes that form the kingdom Plantae; they are predominantly photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with cyanobacteria to produce sugars f ...
, bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
, and humans
Humans (''Homo sapiens'') or modern humans are the most common and widespread species of primate, and the last surviving species of the genus ''Homo''. They are Hominidae, great apes characterized by their Prehistory of nakedness and clothing ...
. Heat shock proteins are primarily responsible for maintaining the integrity of cellular proteins particularly in response to environmental changes. Stresses such as temperature, concentration imbalance, pH change, and toxins can all induce heat shock proteins to maintain the conformation of the cell’s proteins. HSP60 aids in the folding and conformation maintenance of approximately 15-30% of all cellular proteins. In addition to HSP60’s typical role as a heat shock protein, studies have shown that HSP60 plays an important role in the transport
Transport (in British English) or transportation (in American English) is the intentional Motion, movement of humans, animals, and cargo, goods from one location to another. Mode of transport, Modes of transport include aviation, air, land tr ...
and maintenance of mitochondrial proteins as well as the transmission and replication of mitochondrial DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
.
Mitochondrial protein transport
HSP60 possesses two main responsibilities with respect to mitochondrial protein transport. It functions tocatalyze
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
the folding of proteins destined for the matrix and maintains protein in an unfolded state for transport across the inner membrane of the mitochondria. Many proteins are targeted for processing in the matrix of the mitochondria but then are quickly exported to other parts of the cell. The hydrophobic portion HSP60 is responsible for maintaining the unfolded conformation of the protein for transmembrane transport. Studies have shown how HSP60 binds to incoming proteins and induces conformational and structural changes. Subsequent changes in ATP concentrations hydrolyze the bonds between the protein and HSP60 which signals the protein to exit the mitochondria. HSP60 is also capable of distinguishing between proteins designated for export and proteins destined to remain in the mitochondrial matrix by looking for an amphiphilic
In chemistry, an amphiphile (), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'', nonpolar) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic c ...
alpha-helix of 15-20 residues. The existence of this sequence signals that the protein is to be exported while the absence signals that the protein is to remain in the mitochondria. The precise mechanism is not yet entirely understood.
DNA metabolism
In addition to its critical role in protein folding, HSP60 is involved in the replication and transmission ofmitochondrial DNA
Mitochondrial DNA (mtDNA and mDNA) is the DNA located in the mitochondrion, mitochondria organelles in a eukaryotic cell that converts chemical energy from food into adenosine triphosphate (ATP). Mitochondrial DNA is a small portion of the D ...
. In extensive studies of HSP60 activity in ''Saccharomyces cerevisiae'', scientists have proposed that HSP60 binds preferentially to the single stranded template DNA strand in a tetradecamer like complex Kaufman, BA. Studies on mitochondria DNA nucleoids in Saccharomyces cerevisiae: identification of bifunctional proteins. ''In Genetics and Development'', UT Southwestern Medical Center at Dallas, Dallas, TX. 241pp.
This tetradecamer complex interacts with other transcriptional elements to serve as a regulatory mechanism for the replication and transmission of mitochondrial DNA. Mutagenic studies have further supported HSP60 regulatory involvement in the replication and transmission of mitochondrial DNA.Mutations
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mitosi ...
in HSP60 increase the levels of mitochondrial DNA and result in subsequent transmission defects.
Cytoplasmic vs mitochondrial HSP60
In addition to the already illustrated structural differences between cytoplasmic and mitochondrial HSP60, there are marked functional differences. Studies have suggested that HSP60 plays a key role in preventingapoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biol ...
in the cytoplasm. The cytoplasmic HSP60 forms a complex with proteins responsible for apoptosis and regulates the activity of these proteins. The cytoplasmic version is also involved in immune
In biology, immunity is the state of being insusceptible or resistant to a noxious agent or process, especially a pathogen or infectious disease. Immunity may occur naturally or be produced by prior exposure or immunization.
Innate and adaptive ...
response and cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
. These two aspects will be elaborated on later. Extremely recent investigations have begun to suggest a regulatory correlation between HSP60 and the glycolytic
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form ...
enzyme, 6- phosphofructokinase-1. Although not much information is available, cytoplasmic HSP60 concentrations have influenced the expression of 6-phosphofructokinase in glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form ...
. Despite these marked differences between the cytoplasmic and mitochondrial form, experimental analysis has shown that the cell is quickly capable of moving cytoplasmic HSP60 into the mitochondria if environmental conditions demand a higher presence of mitochondrial HSP60.
Synthesis and assembly
HSP60 is typically found in the mitochondria and has been found in organelles of endosymbiotic origin. HSP60 monomers form two heptameric rings that bind to the surface of linear proteins and catalyze their folding in an ATP dependent process. HSP60 subunits are encoded by nucleargenes
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
and translated into the cytosol. These subunits then move into the mitochondria where they are processed by other HSP60 molecules. Several studies have shown how HSP60 proteins must be present in the mitochondria for the synthesis and assembly of additional HSP60 components. There is a direct positive correlation between the presence of HSP60 proteins in the mitochondria and the production of additional HSP60 protein complexes.
The kinetics of assembly of HSP60 subunits into the 2-heptameric rings takes two minutes. The subsequent protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
-resistant HSP60 is formed in a half-time of 5–10 minutes. This rapid synthesis indicates that there is an ATP-dependent interaction where the formed HSP60 complex stabilizes the intermediate of the HSP60 assembly complex, effectively serving as a catalyst. The necessity of preexisting HSP60 in order to synthesize additional HSP60 molecules supports the endosymbiotic theory
Symbiogenesis (endosymbiotic theory, or serial endosymbiotic theory) is the leading evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms. The theory holds that mitochondria, plastids such as chloroplasts, and possibl ...
of the origin of mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
. There must have been a rudimentary prokaryotic homologous protein that was capable of similar self-assembly.
Immunological role
As discussed above, HSP60 has generally been known as a chaperonin which assists in protein folding in mitochondria. However, some new research has indicated that HSP60 possibly plays a role in a “danger signal cascade”immune response
An immune response is a physiological reaction which occurs within an organism in the context of inflammation for the purpose of defending against exogenous factors. These include a wide variety of different toxins, viruses, intra- and extracellula ...
. There is also mounting evidence that it plays a role in autoimmune
In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an " autoimmune disease" ...
disease.
Infection
An infection is the invasion of tissue (biology), tissues by pathogens, their multiplication, and the reaction of host (biology), host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmis ...
and disease
A disease is a particular abnormal condition that adversely affects the structure or function (biology), function of all or part of an organism and is not immediately due to any external injury. Diseases are often known to be medical condi ...
are extremely stressful on the cell. When a cell is under stress, it naturally increases the production of stress proteins, including heat shock proteins
Heat shock proteins (HSPs) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including ex ...
such as HSP60. In order for HSP60 to act as a signal it must be present in the extracellular
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions ...
environment. In recent research “it has emerged that…chaperonin 60 can be found on the surface of various prokaryotic
A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' ...
and eukaryotic
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
cells, and can even be released from cells”. According to recent research, many different types of heat shock proteins
Heat shock proteins (HSPs) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including ex ...
are used in immune
In biology, immunity is the state of being insusceptible or resistant to a noxious agent or process, especially a pathogen or infectious disease. Immunity may occur naturally or be produced by prior exposure or immunization.
Innate and adaptive ...
response signaling, but it appears that different proteins act and respond differently to other signaling molecules. HSP60 has been shown to be released from specific cells like peripheral blood mononuclear cell
A peripheral blood mononuclear cell (PBMC) is any peripheral blood cell having a round Cell nucleus, nucleus. These cells consist of lymphocytes (T cells, B cells, NK cells) and monocytes, whereas erythrocytes and platelets have no nuclei, and gr ...
s (PBMCs) when there are lipopolysaccharides (LPS) or GroEL present. This suggests that the cell has different receptors
Receptor may refer to:
*Sensory receptor, in physiology, any neurite structure that, on receiving environmental stimuli, produces an informative nerve impulse
*Receptor (biochemistry), in biochemistry, a protein molecule that receives and responds ...
and responses to human and bacterial HSP60. In addition, it has been shown that HSP60 has the capability “of activating monocytes
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also i ...
, macrophages
Macrophages (; abbreviated MPhi, φ, MΦ or MP) are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris and foreign substances, which do not have proteins that ...
and dendritic
Dendrite derives from the Greek word "dendron" meaning ( "tree-like"), and may refer to:
Biology
*Dendrite, a branched projection of a neuron
*Dendrite (non-neuronal), branching projections of certain skin cells and immune cells
Physical
*Dendri ...
cells…and also of inducing secretion of a wide range of cytokines
Cytokines () are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling.
Cytokines are produced by a broad range of cells, including immune cells like macrophages, B cell, B lymphocytes, T cell, T lymphocytes ...
.” The fact that HSP60 responds to other signal molecules like LPS or GroEL and has the ability to activate certain types of cells supports the idea that HSP60 is part of a danger signal cascade which is involved in activating an immune response.
There is however, a twist in the immunological role of HSP60. As mentioned above, there are two different types of HSP60 proteins, bacterial as well as mammalian. Since they are very similar in sequence, bacterial HSP60 wouldn’t be expected to cause a large immune response in humans. The immune system is “designed to ignore ‘self’, that is, host constituents; however, paradoxically, this is not the case with chaperonins”. It has been found that many anti-chaperonin antibodies exist and are associated with many autoimmune diseases. According to Ranford, et al. experiments have been performed which have shown that antibodies
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that caus ...
which are “generated by a human host after exposure to bacterial chaperonin 60 proteins” can cross-react with human chaperonin 60 proteins. Bacterial HSP60 is causing the immune system to create anti-chaperonin antibodies, even though bacterial and human HSP60 have similar protein sequences. These new antibodies are then recognizing and attacking human HSP60 which causes an autoimmune disease. This suggests that HSP60 may play a role in autoimmunity
In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an " autoimmune disease ...
, however more research needs to be done in order to discover more completely its role in this disease.
Stress response
HSP60, as a mitochondrial protein, has been shown to be involved in stress response as well. The heat shock response is a homeostatic mechanism that protects a cell from damage by upregulating the expression of genes that code for HSP60. The upregulation of HSP60 production allows for the maintenance of other cellular processes occurring in the cell, especially during stressful times. In one experiment, investigators treated various mice withL-DOPA
-DOPA, also known as -3,4-dihydroxyphenylalanine and used medically as levodopa, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize -DO ...
and discovered significant upregulation of HSP60 expression in the mitochondria and HSP70
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms and play crucial roles in the development of can ...
expression in the cytoplasm. Researchers concluded that the heat shock signal pathway serves as “the basic mechanism of defense against neurotoxicity
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specifical ...
elicited by free radical oxygen and nitrogen species produced in aging and neurodegenerative disorders”.
Several studies have shown that HSP60 and other heat shock proteins are necessary for cellular survival under toxic or stressful circumstances.
Relationship to cancer
Human Hsp60, the product of the HSPD1 gene, is a Group I mitochondrial chaperonin, phylogenetically related to bacterial GroEL. Recently, the presence of Hsp60 outside the mitochondria and outside the cell, e.g. in circulating blood, has been reported Although it is assumed that Hsp60 extra-mitochondrial molecule is identical to the mitochondrial one, this has not yet been fully elucidated. Despite the increasing amount of experimental evidences showing Hsp60 outside the cell, it is not yet clear how general this process is and what are the mechanisms responsible for Hsp60 translocation outside the cell. Neither of these questions has been definitively answered, whereas there is some information regarding extracellular Hsp70. This chaperone was also classically regarded as an intracellular protein like Hsp60, but in the last few years considerable evidences showed its pericellular and extracellular residence HSP60 has been shown to influenceapoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biol ...
in tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
cells which seems to be associated with a change in expression levels. There is some inconsistency in that some research shows a positive expression while other research shows a negative expression, and it seems to depend on the type of cancer. There are different hypotheses to explain the effects of positive versus negative expression. Positive expression seems to inhibit “ apoptotic and necrotic
Necrosis () is a form of cell injury which results in the premature death of cells in living tissue by autolysis. The term "necrosis" came about in the mid-19th century and is commonly attributed to German pathologist Rudolf Virchow, who is ...
cell death” while negative expression is thought to play a part “in activation of apoptosis”.
As well as influencing apoptosis, HSP60 changes in expression level have been shown to be “useful new biomarkers for diagnostic and prognostic purposes.” According to Lebret et al., a loss of HSP60 expression “indicates a poor prognosis and the risk of developing tumor infiltration” specifically with bladder
The bladder () is a hollow organ in humans and other vertebrates that stores urine from the kidneys. In placental mammals, urine enters the bladder via the ureters and exits via the urethra during urination. In humans, the bladder is a distens ...
carcinomas, but that does not necessarily hold true for other types of cancers. For example, ovarian tumors research has shown that over expression is correlated with a better prognosis while a decreased expression is correlated with an aggressive tumor. All this research indicates that it may be possible for HSP60 expression to be used in predicting survival for certain types of cancer and therefore may be able to identify patients who could benefit from certain treatments.
Mechanism
Within the cell, the process of GroEL/ES mediated protein folding involves multiple rounds of binding, encapsulation, and release of substrate protein. Unfolded substrate proteins bind to a hydrophobic binding patch on the interior rim of the open cavity of GroEL, forming a binary complex with the chaperonin. Binding of substrate protein in this manner, in addition to binding of ATP, induces aconformational change
In biochemistry, a conformational change is a change in the shape of a macromolecule, often induced by environmental factors.
A macromolecule is usually flexible and dynamic. Its shape can change in response to changes in its environment or othe ...
that allows association of the binary complex with a separate lid structure, GroES
Heat shock 10 kDa protein 1 (Hsp10), also known as chaperonin 10 (cpn10) or early-pregnancy factor (EPF), is a protein that in humans is encoded by the ''HSPE1'' gene. The homolog in ''Escherichia coli, E. coli'' is GroES that is a chaperonin ...
. Binding of GroES to the open cavity of the chaperonin
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 b ...
induces the individual subunits of the chaperonin to rotate such that the hydrophobic substrate binding site is removed from the interior of the cavity, causing the substrate protein to be ejected from the rim into the now largely hydrophilic chamber. The hydrophilic environment of the chamber favors the burying of hydrophobic residues of the substrate, inducing substrate folding. Hydrolysis of ATP and binding of a new substrate protein to the opposite cavity sends an allosteric signal causing GroES and the encapsulated protein to be released into the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
. A given protein will undergo multiple rounds of folding, returning each time to its original unfolded state, until the native conformation or an intermediate structure committed to reaching the native state is achieved. Alternatively, the substrate may succumb to a competing reaction, such as misfolding and aggregation with other misfolded proteins.
Thermodynamics
The constricted nature of the interior of the molecular complex strongly favors compact molecular conformations of the substrate protein. Free in solution, long-range, non- polar interactions can only occur at a high cost inentropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
. In the close quarters of the GroEL complex, the relative loss of entropy is much smaller. The method of capture also tends to concentrate the non-polar binding sites separately from the polar sites. When the GroEL non-polar surfaces are removed, the chance that any given non-polar group will encounter a non-polar intramolecular site are much greater than in bulk solution. The hydrophobic sites which were on the outside are gathered together at the top of the ''cis'' domain and bind each other. The geometry of GroEL requires that the polar structures lead, and they envelop the non-polar core as it emerges from the ''trans'' side.
Structure
Structurally, GroEL is a dual-ringed tetradecamer, with both the ''cis'' and ''trans'' rings consisting of seven subunits each. The conformational changes that occur within the central cavity of GroEL cause for the inside of GroEL to become hydrophilic, rather than hydrophobic, and is likely what facilitates protein folding.binding site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may includ ...
s for unfolded protein substrates. Many globular proteins won't bind to the apical domain because their hydrophobic parts are clustered inside, away from the aqueous medium since this is the thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of th ...
ally optimal conformation. Thus, these "substrate sites" will only bind to proteins which are not optimally folded. The apical domain also has binding sites for the Hsp10 monomers of GroES.
The equatorial domain has a slot near the hinge point for binding ATP, as well as two attachment points for the other half of the GroEL molecule. The rest of the equatorial section is moderately hydrophilic.
The addition of ATP and GroES has a drastic effect on the conformation of the ''cis'' domain. This effect is caused by flexion
Motion, the process of movement, is described using specific anatomical terminology, anatomical terms. Motion includes movement of Organ (anatomy), organs, joints, Limb (anatomy), limbs, and specific sections of the body. The terminology used de ...
and rotation
Rotation or rotational/rotary motion is the circular movement of an object around a central line, known as an ''axis of rotation''. A plane figure can rotate in either a clockwise or counterclockwise sense around a perpendicular axis intersect ...
at the two hinge points on the Hsp60 monomers. The intermediate domain folds down and inward about 25° on the lower hinge. This effect, multiplied through the cooperative flexing of all monomers, increases the equatorial diameter of the GroEL cage. But the apical domain rotates a full 60° up and out on the upper hinge, and also rotates 90° around the hinge axis. This motion opens the cage very widely at the top of the ''cis'' domain, but completely removes the substrate binding sites from the inside of the cage.
Interactions
GroEL has been shown to interact withGroES
Heat shock 10 kDa protein 1 (Hsp10), also known as chaperonin 10 (cpn10) or early-pregnancy factor (EPF), is a protein that in humans is encoded by the ''HSPE1'' gene. The homolog in ''Escherichia coli, E. coli'' is GroES that is a chaperonin ...
, ALDH2
Aldehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ''ALDH2'' gene located on chromosome 12. ALDH2 belongs to the aldehyde dehydrogenase family of enzymes. Aldehyde dehydrogenase is the second enzyme of the majo ...
, Caspase 3
Caspase-3 is a caspase protein that interacts with caspase-8 and caspase-9. It is encoded by the ''CASP3'' gene. ''CASP3'' orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are als ...
and Dihydrofolate reductase
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in one-carbon transfer chemistry. ...
.
Phage T4 morphogenesis
Thegene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
s of bacteriophage (phage) T4 that encode protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s with a role in determining phage T4 structure were identified using conditional lethal mutant
In biology, and especially in genetics, a mutant is an organism or a new genetic character arising or resulting from an instance of mutation, which is generally an alteration of the DNA sequence of the genome or chromosome of an organism. It i ...
s. Most of these proteins proved to be either major or minor structural components of the completed phage particle. However among the gene products (gps) necessary for phage assembly, Snustad identified a group of gps that act catalytically rather than being incorporated themselves into the phage structure. These catalytic gps included gp31. The bacterium ''E. coli'' is the host for phage T4, and the phage encoded gp31 protein appears to be functionally homologous to ''E. coli'' chaparone protein GroES and able to substitute for it in the assembly of phage T4 virions during infection. The role of the phage encoded gp31 protein appears be to interact with the ''E. coli'' host encoded GroEL protein to assist in the correct folding and assembly of the major phage head capsid protein of the phage, gp23.
See also
*Chaperonin
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 b ...
* Heat shock protein
Heat shock proteins (HSPs) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including ex ...
References
Further reading
* * * * * * * * * * * * * * * * * * * *External links
* * (No rights reserved)3D macromolecular structures of GroEL in EMDB
{{Chaperones Moonlighting proteins Protein complexes Molecular chaperones