Gold (Chet Faker Song) on:
[Wikipedia]
[Google]
[Amazon]
Gold is a

 Gold is the most
Gold is the most

 Whereas most metals are gray or silvery white, gold is slightly reddish-yellow. This color is determined by the frequency of
Whereas most metals are gray or silvery white, gold is slightly reddish-yellow. This color is determined by the frequency of
2 Au + 3 F2 -> atop\Delta2 AuF3
2 Au + 3 Cl2 -> atop\Delta2 AuCl3
2 Au + 2 Br2 -> atop\DeltaAuBr3 + AuBr
2 Au + I2 -> atop\Delta2 AuI
Gold does not react with sulfur directly, but 2 Au + 6 H2SeO4 ->
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has chemical symbol
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist ...
Au (from Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
) and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
79. In its pure form, it is a bright
Bright may refer to:
Common meanings
*Bright, an adjective meaning giving off or reflecting illumination; see Brightness
*Bright, an adjective meaning someone with intelligence
People
* Bright (surname)
* Bright (given name)
*Bright, the stage na ...
, slightly orange-yellow, dense, soft, malleable
Ductility refers to the ability of a material to sustain significant plastic deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversi ...
, and ductile
Ductility refers to the ability of a material to sustain significant plastic deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversi ...
metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
. Chemically, gold is a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
, a group 11 element
Group 11, by modern IUPAC numbering, is a group (periodic table), group of chemical elements in the periodic table, consisting of copper (Cu), silver (Ag), gold (Au), and roentgenium (Rg), although no chemical experiments have yet been carried ...
, and one of the noble metal
A noble metal is ordinarily regarded as a metallic chemical element, element that is generally resistant to corrosion and is usually found in nature in its native element, raw form. Gold, platinum, and the other platinum group metals (ruthenium ...
s. It is one of the least reactive
Reactive may refer to:
*Generally, capable of having a reaction (disambiguation)
*An adjective abbreviation denoting a bowling ball coverstock made of reactive resin
*Reactivity (chemistry)
*Reactive mind
*Reactive programming
See also
*Reactanc ...
chemical elements, being the second-lowest in the reactivity series
In chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their "reactivity" from highest to lowest. It is used to summarize inform ...
. It is solid under standard conditions
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
.
Gold often occurs in free element
In chemistry, a free element is a chemical element that is not combined with or chemically bonded to other elements. Examples of elements which can occur as free elements include the molecular oxygen (O) and carbon as diamond or graphite.A. Earn ...
al (native state
In biochemistry, the native state of a protein or nucleic acid is its properly Protein folding, folded and/or assembled form, which is operative and functional. The native state of a biomolecule may possess all four levels of biomolecular structu ...
), as nuggets or grains, in rocks
In geology, rock (or stone) is any naturally occurring solid mass or aggregate of minerals or mineraloid matter. It is categorized by the minerals included, its chemical composition, and the way in which it is formed. Rocks form the Earth's ...
, veins
Veins () are blood vessels in the circulatory system of humans and most other animals that carry blood towards the heart. Most veins carry deoxygenated blood from the tissues back to the heart; exceptions are those of the pulmonary and fetal c ...
, and alluvial deposit
Alluvium (, ) is loose clay, silt, sand, or gravel that has been deposited by running water in a stream bed, on a floodplain, in an alluvial fan or beach, or in similar settings. Alluvium is also sometimes called alluvial deposit. Alluvium is ...
s. It occurs in a solid solution
A solid solution, a term popularly used for metals, is a homogeneous mixture of two compounds in solid state and having a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solutio ...
series with the native element silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
(as in electrum
Electrum is a naturally occurring alloy of gold and silver, with trace amounts of copper and other metals. Its color ranges from pale to bright yellow, depending on the proportions of gold and silver. It has been produced artificially and is ...
), naturally alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
ed with other metals like copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
and palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
, and mineral inclusion
In mineralogy, an inclusion is any material trapped inside a mineral during its formation. In gemology, it is an object enclosed within a gemstone or reaching its surface from the interior. According to James Hutton's law of inclusions, fragment ...
s such as within pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
. Less commonly, it occurs in minerals as gold compounds, often with tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
(gold telluride
Gold chalcogenides are compounds formed between gold and one of the chalcogens, elements from group 16 of the periodic table: oxygen, sulfur, selenium, or tellurium.
* Gold(III) oxide, Au2O3. Decomposes into gold and oxygen above 160 °C, and ...
s).
Gold is resistant to most acids, though it does dissolve in aqua regia
Aqua regia (; from Latin, "regal water" or "royal water") is a mixture of nitric acid and hydrochloric acid, optimally in a molar concentration, molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but i ...
(a mixture of nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
and hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
), forming a soluble tetrachloroaurate
Chloroauric acid is an inorganic compound with the chemical formula . It forms hydrates . Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar anion. Often chloroauric acid is handled as a solutio ...
anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
. Gold is insoluble in nitric acid alone, which dissolves silver and base metal
A base metal is a common and inexpensive metal, as opposed to a precious metal such as gold or silver. In numismatics, coins often derived their value from the precious metal content; however, base metals have also been used in coins in the past ...
s, a property long used to refine
Refining is the process of List of purification methods in chemistry, purification of a (1) chemical compound, substance or a (2) Theory of Forms, form. The term is usually used of a natural resource that is almost in a usable form, but which is m ...
gold and confirm the presence of gold in metallic substances, giving rise to the term "acid test
An acid test is a qualitative chemical or metallurgical assay utilizing acid. Historically, it often involved the use of a robust acid to distinguish gold from base metals. Figuratively, the term represents any definitive test for attributes, suc ...
". Gold dissolves in alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The ...
solutions of cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
, which are used in mining
Mining is the Resource extraction, extraction of valuable geological materials and minerals from the surface of the Earth. Mining is required to obtain most materials that cannot be grown through agriculture, agricultural processes, or feasib ...
and electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric cur ...
. Gold also dissolves in mercury, forming amalgam
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital, an in ...
alloys, and as the gold acts simply as a solute, this is not a chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
.
A relatively rare element, gold is a precious metal
Precious metals are rare, naturally occurring metallic chemical elements of high Value (economics), economic value. Precious metals, particularly the noble metals, are more corrosion resistant and less reactivity (chemistry), chemically reac ...
that has been used for coinage
Coinage may refer to:
* Coins, standardized as currency
* Coining (mint), the process of manufacturing coins
* '' COINage'', a numismatics magazine
* Tin coinage, a tax on refined tin
* Coinage, a protologism or neologism
In linguistics, a neolo ...
, jewelry
Jewellery (or jewelry in American English) consists of decorative items worn for personal adornment such as brooches, ring (jewellery), rings, necklaces, earrings, pendants, bracelets, and cufflinks. Jewellery may be attached to the body or the ...
, and other works of art
A work of art, artwork, art piece, piece of art or art object is an artistic creation of aesthetic value. Except for "work of art", which may be used of any work regarded as art in its widest sense, including works from literature ...
throughout recorded history
Recorded history or written history describes the historical events that have been recorded in a written form or other documented communication which are subsequently evaluated by historians using the historical method. For broader world h ...
. In the past, a gold standard
A gold standard is a backed currency, monetary system in which the standard economics, economic unit of account is based on a fixed quantity of gold. The gold standard was the basis for the international monetary system from the 1870s to the ...
was often implemented as a monetary policy
Monetary policy is the policy adopted by the monetary authority of a nation to affect monetary and other financial conditions to accomplish broader objectives like high employment and price stability (normally interpreted as a low and stable rat ...
. Gold coins ceased to be minted as a circulating currency in the 1930s, and the world gold standard was abandoned for a fiat currency
Fiat money is a type of government-issued currency that is not backed by a precious metal, such as gold or silver, nor by any other tangible asset or commodity. Fiat currency is typically designated by the issuing government to be legal tender, ...
system after the Nixon shock measures of 1971.
In 2023, the world's largest gold producer was China, followed by Russia and Australia. , a total of around 201,296 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the s ...
s of gold exist above ground. If all of this gold were put together into a cube shape, each of its sides would measure . The world's consumption of new gold produced is about 50% in jewelry, 40% in investment
Investment is traditionally defined as the "commitment of resources into something expected to gain value over time". If an investment involves money, then it can be defined as a "commitment of money to receive more money later". From a broade ...
s, and 10% in industry
Industry may refer to:
Economics
* Industry (economics), a generally categorized branch of economic activity
* Industry (manufacturing), a specific branch of economic activity, typically in factories with machinery
* The wider industrial sector ...
. Gold's high malleability, ductility, resistance to corrosion and most other chemical reactions, as well as conductivity of electricity have led to its continued use in corrosion-resistant electrical connector
Components of an electrical circuit are electrically connected if an electric current can run between them through an electrical conductor. An electrical connector is an electromechanical device used to create an electrical connection between ...
s in all types of computerized devices (its chief industrial use). Gold is also used in infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
shielding, the production of colored glass
The appearance of different colors in glass is largely due to the way light interacts with the materials it contains. In an extremely pure glass, without impurities such as bubbles, coloring ions, or crystalline and nano-sized phases, all visible l ...
, gold leaf
upA gold nugget of 5 mm (0.2 in) in diameter (bottom) can be expanded through hammering into a gold foil of about 0.5 m2 (5.4 sq ft). The Japan.html" ;"title="Toi gold mine museum, Japan">Toi gold mine museum, Japan.
Gold leaf is gold that has ...
ing, and tooth restoration. Certain gold salts
Gold-containing drugs are pharmaceuticals that contain gold. Sometimes these species are referred to as "gold salts". "Chrysotherapy" and "aurotherapy" are the applications of gold compounds to medicine. Research on the medicinal effects of g ...
are still used as anti-inflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation, fever or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs reduce pain by inhibiting mechan ...
agents in medicine.
Characteristics

 Gold is the most
Gold is the most malleable
Ductility refers to the ability of a material to sustain significant plastic deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversi ...
of all metals. It can be drawn into a wire of single-atom width, and then stretched considerably before it breaks. Such nanowires distort via the formation, reorientation, and migration of dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to sli ...
s and crystal twins without noticeable hardening. A single gram of gold can be beaten into a sheet of , and an avoirdupois ounce
The ounce () is any of several different units of mass, weight, or volume and is derived almost unchanged from the , an Ancient Roman unit of measurement.
The avoirdupois ounce (exactly ) is avoirdupois pound; this is the United States cu ...
into . Gold leaf can be beaten thin enough to become semi-transparent. The transmitted light appears greenish-blue because gold strongly reflects yellow and red. Such semi-transparent sheets also strongly reflect infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
light, making them useful as infrared (radiant heat) shields in the visors of heat-resistant suits and in sun visors for spacesuit
A space suit (or spacesuit) is an environmental suit used for protection from the harsh Space environment, environment of outer space, mainly from its Vacuum (outer space), vacuum as a highly specialized pressure suit, but also its temperatu ...
s. Gold is a good conductor of heat and electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
.
Gold has a density of 19.3 g/cm3, almost identical to that of tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
at 19.25 g/cm3; as such, tungsten has been used in the counterfeiting
A counterfeit is a fake or unauthorized replica of a genuine product, such as money, documents, designer items, or other valuable goods. Counterfeiting generally involves creating an imitation of a genuine item that closely resembles the original ...
of gold bar
A gold bar, also known as gold bullion or a gold ingot, is a quantity of refined metallic gold that can be shaped in various forms, produced under standardized conditions of manufacture, labeling, and record-keeping. Larger varieties of gold ...
s, such as by plating a tungsten bar with gold. By comparison, the density of lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
is 11.34 g/cm3, and that of the densest element, osmium
Osmium () is a chemical element; it has Symbol (chemistry), symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a Abundance of elements in Earth's crust, trace element in a ...
, is .
Color
plasma oscillation
Plasma oscillations, also known as Langmuir waves (after Irving Langmuir), are rapid oscillations of the electron density in conducting media such as plasmas or metals in the ultraviolet region. The oscillations can be described as an instability ...
s among the metal's valence electrons, in the ultraviolet range for most metals but in the visible range for gold due to relativistic effects affecting the orbitals around gold atoms. Similar effects impart a golden hue to metallic caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
.
Common colored gold alloys include the distinctive eighteen-karat rose gold
Colored Gold is the name given to any gold that has been treated using techniques to change its natural color. Pure gold is slightly reddish yellow in color, but colored gold can come in a variety of different colors by alloying it with different ...
created by the addition of copper. Alloys containing palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
or nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
are also important in commercial jewelry as these produce white gold alloys. Fourteen-karat gold-copper alloy is nearly identical in color to certain bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals (such as phosphorus) or metalloid ...
alloys, and both may be used to produce police and other badge
A badge is a device or accessory, often containing the insignia of an organization, which is presented or displayed to indicate some feat of service, a special accomplishment, a symbol of authority granted by taking an oath (e.g., police and fir ...
s. Fourteen- and eighteen-karat gold alloys with silver alone appear greenish-yellow and are referred to as green gold
Electrum is a naturally occurring alloy of gold and silver, with trace amounts of copper and other metals. Its color ranges from pale to bright yellow, depending on the proportions of gold and silver. It has been produced artificially and is ...
. Blue gold can be made by alloying with iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, and purple gold can be made by alloying with aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
. Less commonly, addition of manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
, indium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
, and other elements can produce more unusual colors of gold for various applications.
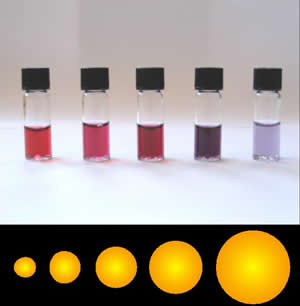
Colloidal gold
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red (for spherical particles less than 100 nm) or blue-purple (for larger spherical partic ...
, used by electron-microscopists, is red if the particles are small; larger particles of colloidal gold are blue.
Isotopes
Gold has only one stableisotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
, , which is also its only naturally occurring isotope, so gold is both a mononuclidic and monoisotopic element
A monoisotopic element is an element which has only a single stable isotope (nuclide). There are 26 such elements, as listed.
Stability is experimentally defined for chemical elements, as there are a number of stable nuclides with atomic number ...
. Thirty-six radioisotopes
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
have been synthesized, ranging in atomic mass
Atomic mass ( or ) is the mass of a single atom. The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the electrons and nuclear binding energy. The atomic mass of atoms, ...
from 169 to 205. The most stable of these is with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 186.1 days. The least stable is , which decays by proton emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a atomic nucleus, nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay ...
with a half-life of 30 μs. Most of gold's radioisotopes with atomic masses below 197 decay by some combination of proton emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a atomic nucleus, nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay ...
, α decay, and β+ decay. The exceptions are , which decays by electron capture, and , which decays most often by electron capture (93%) with a minor β− decay path (7%). All of gold's radioisotopes with atomic masses above 197 decay by β− decay.
At least 32 nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of ...
s have also been characterized, ranging in atomic mass from 170 to 200. Within that range, only , , , , and do not have isomers. Gold's most stable isomer is with a half-life of 2.27 days. Gold's least stable isomer is with a half-life of only 7 ns. has three decay paths: β+ decay, isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 s ...
, and alpha decay. No other isomer or isotope of gold has three decay paths.
Synthesis
The possible production of gold from a more common element, such aslead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
, has long been a subject of human inquiry, and the ancient and medieval discipline of alchemy
Alchemy (from the Arabic word , ) is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practised in China, India, the Muslim world, and Europe. In its Western form, alchemy is first ...
often focused on it; however, the transmutation of the chemical elements did not become possible until the understanding of nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies th ...
in the 20th century. The first synthesis of gold was conducted by Japanese physicist Hantaro Nagaoka
was a Japanese physicist and a pioneer of Japanese physics during the Meiji period.
Life
Nagaoka was born in Nagasaki, Japan on August 19, 1865 and educated at the University of Tokyo. After graduating with a degree in physics in 1887, Naga ...
, who synthesized gold from mercury in 1924 by neutron bombardment. An American team, working without knowledge of Nagaoka's prior study, conducted the same experiment in 1941, achieving the same result and showing that the isotopes of gold
Gold (79Au) has one stable isotope, 197Au, and 40 radioisotopes, with 195Au being the most stable with a half-life of 186 days. Gold is currently considered the heaviest monoisotopic element. Bismuth formerly held that distinction until alpha-de ...
produced by it were all radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
. In 1980, Glenn Seaborg
Glenn Theodore Seaborg ( ; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
transmuted several thousand atoms of bismuth into gold at the Lawrence Berkeley Laboratory. Gold can be manufactured in a nuclear reactor, but doing so is highly impractical and would cost far more than the value of the gold that is produced.
Chemistry
Although gold is the most noble of the noble metals, it still forms many diverse compounds. Theoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s such as thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have ...
s, thiolate
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s, and organophosphine
Organophosphines are organophosphorus compounds with the formula PR''n''H3−''n'', where R is an organic substituent. These compounds can be classified according to the value of ''n'': primary phosphines (''n'' = 1), secondary phosphin ...
s. Au(I) compounds are typically linear. A good example is , which is the soluble form of gold encountered in mining. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.
Au(III) (referred to as auric) is a common oxidation state, and is illustrated by gold(III) chloride
Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula . The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It ...
, . The gold atom centers in Au(III) complexes, like other d8 compounds, are typically square planar
In chemistry, the square planar molecular geometry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the co ...
, with chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s that have both covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
and ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic character. Gold(I,III) chloride
Gold(I,III) chloride is the inorganic compound with the chemical formula Au4 Cl8. It is a mixed valence compound as it contains gold in two oxidation states; square-planar gold(III) and almost linear gold(I). The compound, which is black, is pho ...
is also known, an example of a mixed-valence complex
Mixed valence complexes contain an element which is present in more than one oxidation state. Well-known mixed valence compounds include the Creutz–Taube complex, Prussian blue, and molybdenum blue. Many solids are mixed-valency including ...
.
Gold does not react with oxygen at any temperature and, up to 100 °C, is resistant to attack from ozone:
Some free halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s react to form the corresponding gold halides. Gold is strongly attacked by fluorine at dull-red heat to form gold(III) fluoride
Gold(III) fluoride is an inorganic compound of gold and flourine with the molecular formula . It is an orange solid that sublimes at 300 °C. It is a powerful fluorinating agent. It is very sensitive to moisture, yielding gold(III) hydroxi ...
. Powdered gold reacts with chlorine at 180 °C to form gold(III) chloride
Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula . The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It ...
. Gold reacts with bromine at 140 °C to form a combination of gold(III) bromide
Gold(III) bromide is a dark-red to black crystalline solid.Macintyre, J. E. (ed.) ''Dictionary of Inorganic Compounds''; Chapman & Hall: London, 1992; vol. 1, pp. 121Greenwood, N.N.; Earnshaw, A. ''Chemistry of the Elements''; Butterworth-Heineman ...
and gold(I) bromide AuBr, but reacts very slowly with iodine to form gold(I) iodide
Gold monoiodide is the inorganic compound of gold and iodine with the formula AuI. It can be synthesized by dissolving gold powder in an aqueous solution of iodine and potassium iodide. With Lewis bases, AuI reacts to give numerous complexes.
P ...
AuI:
gold(III) sulfide
Gold(III) sulfide or auric sulfide is an inorganic compound with the formula . Auric sulfide has been described as a black and amorphous solid. Only the amorphous phase has been produced, and the only evidence of existence is based on thermal anal ...
can be made by passing hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
through a dilute solution of gold(III) chloride or chlorauric acid
Chloroauric acid is an inorganic compound with the chemical formula . It forms hydrates . Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar anion. Often chloroauric acid is handled as a solutio ...
.
Unlike sulfur, phosphorus reacts directly with gold at elevated temperatures to produce gold phosphide Gold phosphides are inorganic compounds of gold and phosphorus. The only known gold phosphide is a metastable gold(I) polyphosphide with the formula .
Older texts sometimes refer to a binary auric phosphide ; this hypothetical compound has not be ...
(Au2P3).
Gold readily dissolves in mercury at room temperature to form an amalgam
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital, an in ...
, and forms alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s with many other metals at higher temperatures. These alloys can be produced to modify the hardness and other metallurgical properties, to control melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
or to create exotic colors.
Gold is unaffected by most acids. It does not react with hydrofluoric, hydrochloric
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastr ...
, hydrobromic, hydriodic, sulfuric
Sulfur (American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form ...
, or nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
. It does react with selenic acid
Selenic acid is the inorganic compound with the formula . It is an oxoacid of selenium, and its structure is more accurately described as . It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the pr ...
, and is dissolved by aqua regia
Aqua regia (; from Latin, "regal water" or "royal water") is a mixture of nitric acid and hydrochloric acid, optimally in a molar concentration, molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but i ...
, a 1:3 mixture of nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
and hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
. Nitric acid oxidizes the metal to +3 ions, but only in minute amounts, typically undetectable in the pure acid because of the chemical equilibrium of the reaction. However, the ions are removed from the equilibrium by hydrochloric acid, forming ions, or chloroauric acid
Chloroauric acid is an inorganic compound with the chemical formula . It forms hydrates . Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar anion. Often chloroauric acid is handled as a solutio ...
, thereby enabling further oxidation:
atop
{{Short pages monitor Nickel is toxic, and its release from nickel white gold is controlled by legislation in Europe. Palladium-gold alloys are more expensive than those using nickel. High-karat white gold alloys are more resistant to corrosion than are either pure silver or sterling silver. The Japanese craft of Mokume-gane exploits the color contrasts between laminated colored gold alloys to produce decorative wood-grain effects.
By 2014, the gold jewelry industry was escalating despite a dip in gold prices. Demand in the first quarter of 2014 pushed turnover to $23.7 billion according to a
 Gold alloys are used in restorative dentistry, especially in tooth restorations, such as Crown (dental restoration), crowns and permanent bridge (dentistry), bridges. The gold alloys' slight malleability facilitates the creation of a superior molar mating surface with other teeth and produces results that are generally more satisfactory than those produced by the creation of porcelain crowns. The use of gold crowns in more prominent teeth such as incisors is favored in some cultures and discouraged in others.
Gold alloys are used in restorative dentistry, especially in tooth restorations, such as Crown (dental restoration), crowns and permanent bridge (dentistry), bridges. The gold alloys' slight malleability facilitates the creation of a superior molar mating surface with other teeth and produces results that are generally more satisfactory than those produced by the creation of porcelain crowns. The use of gold crowns in more prominent teeth such as incisors is favored in some cultures and discouraged in others.
 * Gold produces a deep, intense red color when used as a coloring agent in cranberry glass.
* In photography, gold toners are used to shift the color of silver bromide black-and-white prints towards brown or blue tones, or to increase their stability. Used on sepia tone, sepia-toned prints, gold toners produce red tones. Kodak published formulas for several types of gold toners, which use gold as the chloride.
* Gold is a good reflector of electromagnetic radiation such as
* Gold produces a deep, intense red color when used as a coloring agent in cranberry glass.
* In photography, gold toners are used to shift the color of silver bromide black-and-white prints towards brown or blue tones, or to increase their stability. Used on sepia tone, sepia-toned prints, gold toners produce red tones. Kodak published formulas for several types of gold toners, which use gold as the chloride.
* Gold is a good reflector of electromagnetic radiation such as
 * Bulk leach extractable gold, for sampling ores
* Chrysiasis (dermatological condition)
* Digital gold currency, form of electronic currency
* GFMS business consultancy
* Gold fingerprinting, use impurities to identify an alloy
*
* Bulk leach extractable gold, for sampling ores
* Chrysiasis (dermatological condition)
* Digital gold currency, form of electronic currency
* GFMS business consultancy
* Gold fingerprinting, use impurities to identify an alloy
*
online
* Bernstein, Peter L. ''The Power of Gold: The History of an Obsession'' (2000
online
* Brands, H.W. ''The Age of Gold: The California Gold Rush and the New American Dream'' (2003
excerpt
* Buranelli, Vincent. ''Gold : an illustrated history'' (1979
online
wide-ranging popular history * Cassel, Gustav. "The restoration of the gold standard." ''Economica'' 9 (1923): 171–185
online
* Eichengreen, Barry. ''Golden Fetters: The Gold Standard and the Great Depression, 1919–1939'' (Oxford UP, 1992). * Ferguson, Niall. ''The Ascent of Money – Financial History of the World'' (2009
online
* Hart, Matthew
Gold: The Race for the World's Most Seductive Metal
''Gold : the race for the world's most seductive metal", New York: Simon & Schuster, 2013. '' * * Kwarteng, Kwasi. ''War and Gold: A Five-Hundred-Year History of Empires, Adventures, and Debt'' (2014
online
* Vilar, Pierre. ''A History of Gold and Money, 1450–1920'' (1960)
online
* Vilches, Elvira. ''New World Gold: Cultural Anxiety and Monetary Disorder in Early Modern Spain'' (2010).
Chemistry in its element podcast
(MP3) from the Royal Society of Chemistry's Chemistry World
Gold
www.rsc.org
at ''The Periodic Table of Videos'' (University of Nottingham)
''Getting Gold'' 1898 book
www.lateralscience.co.uk * , www.epa.gov
Gold element information
– rsc.org {{Authority control Gold, Chemical elements Transition metals Noble metals Precious metals Cubic minerals Minerals in space group 225 Dental materials Electrical conductors Native element minerals E-number additives Symbols of Alaska Symbols of California Chemical elements with face-centered cubic structure Coinage metals and alloys Symbols of Victoria
World Gold Council
The World Gold Council is an international trade association for the gold industry. It is headquartered in London and has offices in India, China, Singapore, the UAE and the United States. The organization's members are gold mining companies. ...
report.
Gold solder is used for joining the components of gold jewelry by high-temperature hard soldering or brazing. If the work is to be of hallmarking quality, the gold solder alloy must match the fineness of the work, and alloy formulas are manufactured to color-match yellow and white gold. Gold solder is usually made in at least three melting-point ranges referred to as Easy, Medium and Hard. By using the hard, high-melting point solder first, followed by solders with progressively lower melting points, goldsmiths can assemble complex items with several separate soldered joints. Gold can also be made into gold thread, thread and used in embroidery
Embroidery is the art of decorating Textile, fabric or other materials using a Sewing needle, needle to stitch Yarn, thread or yarn. It is one of the oldest forms of Textile arts, textile art, with origins dating back thousands of years across ...
.
Electronics
Only 10% of the world consumption of new gold produced goes to industry, but by far the most important industrial use for new gold is in fabrication of corrosion-free electrical connectors in computers and other electrical devices. For example, according to the World Gold Council, a typical cell phone may contain 50 mg of gold, worth about three dollars. But since nearly one billion cell phones are produced each year, a gold value of US$2.82 in each phone adds to US$2.82 billion in gold from just this application. (Prices updated to November 2022) Though gold is attacked by free chlorine, its good conductivity and general resistance to oxidation and corrosion in other environments (including resistance to non-chlorinated acids) has led to its widespread industrial use in the electronic era as a thin-layer coating onelectrical connector
Components of an electrical circuit are electrically connected if an electric current can run between them through an electrical conductor. An electrical connector is an electromechanical device used to create an electrical connection between ...
s, thereby ensuring good connection. For example, gold is used in the connectors of the more expensive electronics cables, such as audio, video and USB cables. The benefit of using gold over other connector metals such as tin in these applications has been debated; gold connectors are often criticized by audio-visual experts as unnecessary for most consumers and seen as simply a marketing ploy. However, the use of gold in other applications in electronic sliding contacts in highly humid or corrosive atmospheres, and in use for contacts with a very high failure cost (certain computers, communications equipment, spacecraft, jet aircraft engines) remains very common.
Besides sliding electrical contacts, gold is also used in electrical contacts because of its resistance to corrosion, electrical conductivity, ductile, ductility and lack of toxicity. Switch contacts are generally subjected to more intense corrosion stress than are sliding contacts. Fine gold wires are used to connect semiconductor devices to their packages through a process known as wire bonding.
The concentration of free electrons in gold metal is 5.91×1022 cm−3. Gold is highly electrical conductivity, conductive to electricity and has been used for electrical wiring in some high-energy applications (only silver and copper are more conductive per volume, but gold has the advantage of corrosion resistance). For example, gold electrical wires were used during some of the Manhattan Project's atomic experiments, but large high-current silver wires were used in the calutron isotope separator magnets in the project.
It is estimated that 16% of the world's presently-accounted-for gold and 22% of the world's silver is contained in electronic technology in Japan.
Medicine
There are only two gold compounds currently employed as pharmaceuticals in modern medicine (sodium aurothiomalate and auranofin), used in the treatment of arthritis and other similar conditions in the US due to theiranti-inflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation, fever or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs reduce pain by inhibiting mechan ...
properties. These drugs have been explored as a means to help to reduce the pain and swelling of rheumatoid arthritis, and also (historically) against tuberculosis and some parasites.
Some Western esotericism, esotericists and forms of alternative medicine assign metallic gold a healing power, against the scientific consensus.
Historically, metallic and gold compounds have long been used for medicinal purposes. Gold, usually as the metal, is perhaps the most anciently administered medicine (apparently by shamanic practitioners) and known to Dioscorides. In medieval times, gold was often seen as beneficial for the health, in the belief that something so rare and beautiful could not be anything but healthy.
In the 19th century gold had a reputation as an anxiolytic, a therapy for nervous disorders. Depression (mood), Depression, epilepsy, migraine, and glandular problems such as amenorrhea and impotence were treated, and most notably alcoholism (Keeley, 1897).
The apparent paradox of the actual toxicology of the substance suggests the possibility of serious gaps in the understanding of the action of gold in physiology. Only salts and radioisotopes of gold are of pharmacological value, since elemental (metallic) gold is inert to all chemicals it encounters inside the body (e.g., ingested gold cannot be attacked by stomach acid).
 Gold alloys are used in restorative dentistry, especially in tooth restorations, such as Crown (dental restoration), crowns and permanent bridge (dentistry), bridges. The gold alloys' slight malleability facilitates the creation of a superior molar mating surface with other teeth and produces results that are generally more satisfactory than those produced by the creation of porcelain crowns. The use of gold crowns in more prominent teeth such as incisors is favored in some cultures and discouraged in others.
Gold alloys are used in restorative dentistry, especially in tooth restorations, such as Crown (dental restoration), crowns and permanent bridge (dentistry), bridges. The gold alloys' slight malleability facilitates the creation of a superior molar mating surface with other teeth and produces results that are generally more satisfactory than those produced by the creation of porcelain crowns. The use of gold crowns in more prominent teeth such as incisors is favored in some cultures and discouraged in others.
Colloidal gold
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red (for spherical particles less than 100 nm) or blue-purple (for larger spherical partic ...
preparations (suspensions of gold nanoparticles) in water are intensely red-colored, and can be made with tightly controlled particle sizes up to a few tens of nanometers across by reduction of gold chloride with citrate or ascorbate ions. Colloidal gold is used in research applications in medicine, biology and materials science. The technique of immunogold labeling exploits the ability of the gold particles to adsorb protein molecules onto their surfaces. Colloidal gold particles coated with specific antibodies can be used as probes for the presence and position of antigens on the surfaces of cells. In ultrathin sections of tissues viewed by electron microscope, electron microscopy, the immunogold labels appear as extremely dense round spots at the position of the antigen.
Gold, or alloys of gold and palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
, are applied as conductive coating to biological specimens and other non-conducting materials such as plastics and glass to be viewed in a scanning electron microscope. The coating, which is usually applied by sputtering with an argon plasma (physics), plasma, has a triple role in this application. Gold's very high electrical conductivity drains electric charge, electrical charge to earth, and its very high density provides stopping power for electrons in the electron beam, helping to limit the depth to which the electron beam penetrates the specimen. This improves definition of the position and topography of the specimen surface and increases the Angular resolution, spatial resolution of the image. Gold also produces a high output of secondary emission, secondary electrons when irradiated by an electron beam, and these low-energy electrons are the most commonly used signal source used in the scanning electron microscope.
The isotope gold-198 (half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
2.7 days) is used in nuclear medicine, in some cancer treatments and for treating other diseases.
Cuisine
* Gold can be used in food and has the E number 175. In 2016, the European Food Safety Authority published an opinion on the re-evaluation of gold as a food additive. Concerns included the possible presence of minute amounts of gold nanoparticles in the food additive, and that gold nanoparticles have been shown to be genotoxic in mammalian cells in vitro. * Gold leaf, flake or dust is used on and in some gourmet foods, notably sweets and drinks as decorative ingredient. Gold flake was used by the nobility in medieval Europe as a decoration in food and drinks. * Danziger Goldwasser (German: Gold water of Danzig) or Goldwasser () is a traditional German herbal liqueur produced in what is today Gdańsk, Poland, and Schwabach, Germany, and contains flakes of gold leaf. There are also some expensive (c. $1000) cocktails which contain flakes of gold leaf. However, since metallic gold is inert to all body chemistry, it has no taste, it provides no nutrition, and it leaves the body unaltered. * Vark is a Metal leaf, foil composed of a pure metal that is sometimes gold, and is used for Garnish (food), garnishing sweets in South Asian cuisine.Miscellanea
 * Gold produces a deep, intense red color when used as a coloring agent in cranberry glass.
* In photography, gold toners are used to shift the color of silver bromide black-and-white prints towards brown or blue tones, or to increase their stability. Used on sepia tone, sepia-toned prints, gold toners produce red tones. Kodak published formulas for several types of gold toners, which use gold as the chloride.
* Gold is a good reflector of electromagnetic radiation such as
* Gold produces a deep, intense red color when used as a coloring agent in cranberry glass.
* In photography, gold toners are used to shift the color of silver bromide black-and-white prints towards brown or blue tones, or to increase their stability. Used on sepia tone, sepia-toned prints, gold toners produce red tones. Kodak published formulas for several types of gold toners, which use gold as the chloride.
* Gold is a good reflector of electromagnetic radiation such as infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
and visible spectrum, visible light, as well as radio frequency, radio waves. It is used for the protective coatings on many artificial satellites, in infrared protective faceplates in thermal-protection suits and astronauts' helmets, and in electronic warfare planes such as the EA-6B Prowler.
* Gold is used as the reflective layer on some Gold CD, high-end CDs.
* Automobiles may use gold for heat shielding. McLaren uses gold foil in the engine compartment of its McLaren F1, F1 model.
* Gold can be manufactured so thin that it appears semi-transparent. It is used in some aircraft cockpit windows for Deicing, de-icing or anti-icing by passing electricity through it. The heat produced by the resistance of the gold is enough to prevent ice from forming.
* Gold is attacked by and dissolves in alkaline solutions of potassium or sodium cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
, to form the salt gold cyanide—a technique that has been used in extracting metallic gold from ores in the cyanide process. Gold cyanide is the electrolyte used in commercial electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric cur ...
of gold onto base metals and electroforming.
* Gold chloride (chloroauric acid
Chloroauric acid is an inorganic compound with the chemical formula . It forms hydrates . Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar anion. Often chloroauric acid is handled as a solutio ...
) solutions are used to make colloidal gold by reduction with citrate or ascorbate ions. Gold chloride and gold oxide are used to make cranberry or red-colored glass, which, like colloidal gold suspensions, contains evenly sized spherical gold nanoparticles.
* Gold, when dispersed in nanoparticles, can act as a Heterogeneous gold catalysis, heterogeneous catalyst of chemical reactions.
* In recent years, gold has been used as a symbol of pride by the autism rights movement, as its symbol Au could be seen as similar to the word "Autism spectrum disorder, autism".
Toxicity
Pure metallic (elemental) gold is non-toxic and non-irritating when ingested and is sometimes used as a food decoration in the form ofgold leaf
upA gold nugget of 5 mm (0.2 in) in diameter (bottom) can be expanded through hammering into a gold foil of about 0.5 m2 (5.4 sq ft). The Japan.html" ;"title="Toi gold mine museum, Japan">Toi gold mine museum, Japan.
Gold leaf is gold that has ...
. Metallic gold is also a component of the alcoholic drinks Goldschläger, Gold Strike (drink), Gold Strike, and Goldwasser. Metallic gold is approved as a food additive in the EU (E number, E175 in the Codex Alimentarius). Although the gold ion is toxic, the acceptance of metallic gold as a food additive is due to its relative chemical inertness, and resistance to being corroded or transformed into soluble salts (gold compounds) by any known chemical process which would be encountered in the human body.
Soluble compounds (gold salts
Gold-containing drugs are pharmaceuticals that contain gold. Sometimes these species are referred to as "gold salts". "Chrysotherapy" and "aurotherapy" are the applications of gold compounds to medicine. Research on the medicinal effects of g ...
) such as gold(I,III) chloride, gold chloride are toxic to the liver and kidneys. Common cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
salts of gold such as potassium gold cyanide, used in gold electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric cur ...
, are toxic by virtue of both their cyanide and gold content. There are rare cases of lethal gold poisoning from potassium gold cyanide. Gold toxicity can be ameliorated with chelation therapy with an agent such as dimercaprol.
Gold metal was voted Allergen of the Year in 2001 by the American Contact Dermatitis Society; gold contact allergies affect mostly women. Despite this, gold is a relatively non-potent contact allergen, in comparison with metals like nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
.
A sample of the fungus ''Aspergillus niger'' was found growing from gold mining solution; and was found to contain cyano metal complexes, such as gold, silver, copper, iron and zinc. The fungus also plays a role in the solubilization of heavy metal sulfides.
See also
 * Bulk leach extractable gold, for sampling ores
* Chrysiasis (dermatological condition)
* Digital gold currency, form of electronic currency
* GFMS business consultancy
* Gold fingerprinting, use impurities to identify an alloy
*
* Bulk leach extractable gold, for sampling ores
* Chrysiasis (dermatological condition)
* Digital gold currency, form of electronic currency
* GFMS business consultancy
* Gold fingerprinting, use impurities to identify an alloy
* Gold standard
A gold standard is a backed currency, monetary system in which the standard economics, economic unit of account is based on a fixed quantity of gold. The gold standard was the basis for the international monetary system from the 1870s to the ...
in banking
* List of countries by gold production
* Tumbaga, alloy of gold and copper
* Iron pyrite, fool's gold
* Nordic gold, non-gold copper alloy
References
Further reading
* Bachmann, H. G. ''The lure of gold : an artistic and cultural history'' (2006online
* Bernstein, Peter L. ''The Power of Gold: The History of an Obsession'' (2000
online
* Brands, H.W. ''The Age of Gold: The California Gold Rush and the New American Dream'' (2003
excerpt
* Buranelli, Vincent. ''Gold : an illustrated history'' (1979
online
wide-ranging popular history * Cassel, Gustav. "The restoration of the gold standard." ''Economica'' 9 (1923): 171–185
online
* Eichengreen, Barry. ''Golden Fetters: The Gold Standard and the Great Depression, 1919–1939'' (Oxford UP, 1992). * Ferguson, Niall. ''The Ascent of Money – Financial History of the World'' (2009
online
* Hart, Matthew
Gold: The Race for the World's Most Seductive Metal
''Gold : the race for the world's most seductive metal", New York: Simon & Schuster, 2013. '' * * Kwarteng, Kwasi. ''War and Gold: A Five-Hundred-Year History of Empires, Adventures, and Debt'' (2014
online
* Vilar, Pierre. ''A History of Gold and Money, 1450–1920'' (1960)
online
* Vilches, Elvira. ''New World Gold: Cultural Anxiety and Monetary Disorder in Early Modern Spain'' (2010).
External links
*Chemistry in its element podcast
(MP3) from the Royal Society of Chemistry's Chemistry World
Gold
www.rsc.org
at ''The Periodic Table of Videos'' (University of Nottingham)
''Getting Gold'' 1898 book
www.lateralscience.co.uk * , www.epa.gov
Gold element information
– rsc.org {{Authority control Gold, Chemical elements Transition metals Noble metals Precious metals Cubic minerals Minerals in space group 225 Dental materials Electrical conductors Native element minerals E-number additives Symbols of Alaska Symbols of California Chemical elements with face-centered cubic structure Coinage metals and alloys Symbols of Victoria