Gamma Ray on:
[Wikipedia]
[Google]
[Amazon]
 A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of

 One example of gamma ray production due to radionuclide decay is the decay scheme for cobalt-60, as illustrated in the accompanying diagram. First, decays to excited by
One example of gamma ray production due to radionuclide decay is the decay scheme for cobalt-60, as illustrated in the accompanying diagram. First, decays to excited by

 Due to their penetrating nature, gamma rays require large amounts of shielding mass to reduce them to levels which are not harmful to living cells, in contrast to
Due to their penetrating nature, gamma rays require large amounts of shielding mass to reduce them to levels which are not harmful to living cells, in contrast to
 When a gamma ray passes through matter, the probability for absorption is proportional to the thickness of the layer, the density of the material, and the absorption cross section of the material. The total absorption shows an exponential decrease of intensity with distance from the incident surface:
:
where x is the thickness of the material from the incident surface, μ= ''n''σ is the absorption coefficient, measured in cm−1, ''n'' the number of atoms per cm3 of the material (atomic density) and σ the absorption cross section in cm2.
As it passes through matter, gamma radiation ionizes via three processes:
* The
When a gamma ray passes through matter, the probability for absorption is proportional to the thickness of the layer, the density of the material, and the absorption cross section of the material. The total absorption shows an exponential decrease of intensity with distance from the incident surface:
:
where x is the thickness of the material from the incident surface, μ= ''n''σ is the absorption coefficient, measured in cm−1, ''n'' the number of atoms per cm3 of the material (atomic density) and σ the absorption cross section in cm2.
As it passes through matter, gamma radiation ionizes via three processes:
* The
 Gamma rays provide information about some of the most energetic phenomena in the universe; however, they are largely absorbed by the Earth's atmosphere. Instruments aboard high-altitude balloons and satellites missions, such as the Fermi Gamma-ray Space Telescope, provide our only view of the universe in gamma rays.
Gamma-induced molecular changes can also be used to alter the properties of semi-precious stones, and is often used to change white
Gamma rays provide information about some of the most energetic phenomena in the universe; however, they are largely absorbed by the Earth's atmosphere. Instruments aboard high-altitude balloons and satellites missions, such as the Fermi Gamma-ray Space Telescope, provide our only view of the universe in gamma rays.
Gamma-induced molecular changes can also be used to alter the properties of semi-precious stones, and is often used to change white
 The conventional distinction between X-rays and gamma rays has changed over time. Originally, the electromagnetic radiation emitted by
The conventional distinction between X-rays and gamma rays has changed over time. Originally, the electromagnetic radiation emitted by 
Basic reference on several types of radiation
Radiation Q & A
Radiation information
The Lund/LBNL Nuclear Data Search
– Contains information on gamma-ray energies from isotopes.
The LIVEChart of Nuclides – IAEA
with filter on gamma-ray energy
Health Physics Society Public Education Website
{{Authority control Articles containing video clips Electromagnetic spectrum IARC Group 1 carcinogens Nuclear physics Radiation Radioactivity
electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
arising from high energy interactions like the radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
of atomic nuclei
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the University of Manchester based on the 1909 Geiger–Marsden gold foil experiment. Aft ...
or astronomical events like solar flares. It consists of the shortest wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
electromagnetic waves, typically shorter than those of X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s. With frequencies
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
above 30 exahertz () and wavelengths less than 10 picometers (), gamma ray photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s have the highest photon energy
Photon energy is the energy carried by a single photon. The amount of energy is directly proportional to the photon's electromagnetic frequency and thus, equivalently, is inversely proportional to the wavelength. The higher the photon's frequenc ...
of any form of electromagnetic radiation. Paul Villard, a French chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...
and physicist
A physicist is a scientist who specializes in the field of physics, which encompasses the interactions of matter and energy at all length and time scales in the physical universe. Physicists generally are interested in the root or ultimate cau ...
, discovered gamma radiation in 1900 while studying radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:
* ''electromagnetic radiation'' consisting of photons, such as radio waves, microwaves, infr ...
emitted by radium
Radium is a chemical element; it has chemical symbol, symbol Ra and atomic number 88. It is the sixth element in alkaline earth metal, group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, ...
. In 1903, Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
named this radiation ''gamma rays'' based on their relatively strong penetration of matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic pa ...
; in 1900, he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel
Antoine Henri Becquerel ( ; ; 15 December 1852 – 25 August 1908) was a French nuclear physicist who shared the 1903 Nobel Prize in Physics with Marie and Pierre Curie for his discovery of radioactivity.
Biography
Family and education
Becq ...
) alpha rays
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 atomic nucleus, nucleus. They are generally produced in the process of alpha decay but may ...
and beta rays in ascending order of penetrating power.
Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an electric potential difference of one volt in vacuum. When us ...
(keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lifetimes. The energy spectrum of gamma rays can be used to identify the decaying radionuclides
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
using gamma spectroscopy
Gamma-ray spectroscopy is the ''qualitative'' study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Gamma-ray spectrometry, on the other hand, is the method used to acqu ...
. Very-high-energy gamma ray
A very-high-energy gamma ray (VHEGR) is Gamma ray, gamma radiation with photon energy, photon energies of 100 GeV (gigaelectronvolt) to 100 TeV (teraelectronvolt), i.e., 1011 to 1014 electronvolts. This is approximately equal to
wavelengths betwe ...
s in the 100–1000 teraelectronvolt (TeV) range have been observed from astronomical sources such as the Cygnus X-3
Cygnus X-3 is a high-mass X-ray binary ( HMXB), one of the stronger binary X-ray sources in the sky. It is often considered to be a microquasar, and it is believed to be a compact object in a binary system which is pulling in a stre ...
microquasar
A microquasar, a smaller version of a quasar, is a compact region surrounding a stellar black hole with a mass several times that of its companion star, observable in sufficient details, in our own or nearby galaxy. The matter being pulled from ...
.
Natural sources of gamma rays originating on Earth are mostly a result of radioactive decay and secondary radiation from atmospheric interactions with cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
particles. However, there are other rare natural sources, such as terrestrial gamma-ray flash
A terrestrial gamma-ray flash (TGF), also known as dark lightning, is a burst of gamma rays produced in Earth's atmosphere. TGFs have been recorded to last 0.2 to 3.5 milliseconds, and have energies of up to 20 million electronvolts. It is spe ...
es, which produce gamma rays from electron action upon the nucleus. Notable artificial sources of gamma rays include fission, such as that which occurs in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s, and high energy physics
Particle physics or high-energy physics is the study of fundamental particles and forces that constitute matter and radiation. The field also studies combinations of elementary particles up to the scale of protons and neutrons, while the stu ...
experiments, such as neutral pion decay and nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
.
The energy ranges of gamma rays and X-rays overlap in the electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
, so the terminology for these electromagnetic waves varies between scientific disciplines. In some fields of physics, they are distinguished by their origin: gamma rays are created by nuclear decay while X-rays originate outside the nucleus. In astrophysics
Astrophysics is a science that employs the methods and principles of physics and chemistry in the study of astronomical objects and phenomena. As one of the founders of the discipline, James Keeler, said, astrophysics "seeks to ascertain the ...
, gamma rays are conventionally defined as having photon energies above 100 keV and are the subject of gamma-ray astronomy
Gamma-ray astronomy is a subfield of astronomy where scientists observe and study celestial objects and phenomena in outer space which emit cosmic electromagnetic radiation in the form of gamma rays,Astronomical literature generally hyphena ...
, while radiation below 100 keV is classified as X-rays and is the subject of X-ray astronomy
X-ray astronomy is an observational branch of astronomy which deals with the study of X-ray observation and detection from astronomical objects. X-radiation is absorbed by the Earth's atmosphere, so instruments to detect X-rays must be taken to ...
.
Gamma rays are ionizing radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching ...
and are thus hazardous to life. They can cause DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, ...
s, cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
and tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
s, and at high doses burns and radiation sickness
Acute radiation syndrome (ARS), also known as radiation sickness or radiation poisoning, is a collection of health effects that are caused by being exposed to high amounts of ionizing radiation in a short period of time. Symptoms can start wit ...
. Due to their high penetration power, they can damage bone marrow and internal organs. Unlike alpha and beta rays, they easily pass through the body and thus pose a formidable radiation protection
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposu ...
challenge, requiring shielding made from dense materials such as lead or concrete. On Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
, the magnetosphere
In astronomy and planetary science, a magnetosphere is a region of space surrounding an astronomical object in which charged particles are affected by that object's magnetic field. It is created by a celestial body with an active interior Dynamo ...
protects life from most types of lethal cosmic radiation other than gamma rays.
History of discovery
The first gamma ray source to be discovered was theradioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
process called ''gamma decay''. In this type of decay, an excited nucleus emits a gamma ray almost immediately upon formation.It is now understood that a nuclear isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 s ...
, however, can produce inhibited gamma decay with a measurable and much longer half-life. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium
Radium is a chemical element; it has chemical symbol, symbol Ra and atomic number 88. It is the sixth element in alkaline earth metal, group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, ...
. Villard knew that his described radiation was more powerful than previously described types of rays from radium, which included beta rays, first noted as "radioactivity" by Henri Becquerel
Antoine Henri Becquerel ( ; ; 15 December 1852 – 25 August 1908) was a French nuclear physicist who shared the 1903 Nobel Prize in Physics with Marie and Pierre Curie for his discovery of radioactivity.
Biography
Family and education
Becq ...
in 1896, and alpha rays, discovered as a less penetrating form of radiation by Rutherford, in 1899. However, Villard did not consider naming them as a different fundamental type. Later, in 1903, Villard's radiation was recognized as being of a type fundamentally different from previously named rays by Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
, who named Villard's rays "gamma rays" by analogy with the beta and alpha rays that Rutherford had differentiated in 1899. The "rays" emitted by radioactive elements were named in order of their power to penetrate various materials, using the first three letters of the Greek alphabet: alpha rays as the least penetrating, followed by beta rays, followed by gamma rays as the most penetrating. Rutherford also noted that gamma rays were not deflected (or at least, not deflected) by a magnetic field, another property making them unlike alpha and beta rays.
Gamma rays were first thought to be particles with mass, like alpha and beta rays. Rutherford initially believed that they might be extremely fast beta particles, but their failure to be deflected by a magnetic field indicated that they had no charge. In 1914, gamma rays were observed to be reflected from crystal surfaces, proving that they were electromagnetic radiation. Rutherford and his co-worker Edward Andrade measured the wavelengths of gamma rays from radium, and found they were similar to X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s, but with shorter wavelengths and thus, higher frequency. This was eventually recognized as giving them more energy per photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
, as soon as the latter term became generally accepted. A gamma decay was then understood to usually emit a gamma photon.
Sources
Natural sources of gamma rays on Earth include gamma decay from naturally occurringradioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
s such as potassium-40
Potassium-40 (K) is a long lived and the main naturally occurring radioactive isotope of potassium. Its half-life is 1.25 billion years. It makes up about 0.012% (120 parts-per notation, ppm) of natural potassium.
Potassium-40 undergoes four dif ...
, and also as a secondary radiation from various atmospheric interactions with cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
particles. Natural terrestrial sources that produce gamma rays include lightning strike
A lightning strike or lightning bolt is a lightning event in which an electric discharge takes place between the atmosphere and the ground. Most originate in a cumulonimbus cloud and terminate on the ground, called cloud-to-ground (CG) lightning ...
s and terrestrial gamma-ray flash
A terrestrial gamma-ray flash (TGF), also known as dark lightning, is a burst of gamma rays produced in Earth's atmosphere. TGFs have been recorded to last 0.2 to 3.5 milliseconds, and have energies of up to 20 million electronvolts. It is spe ...
es, which produce high energy emissions from natural high-energy voltages. Gamma rays are produced by a number of astronomical processes in which very high-energy electrons are produced. Such electrons produce secondary gamma rays by the mechanisms of ''bremsstrahlung
In particle physics, bremsstrahlung (; ; ) is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic ...
'', inverse Compton scattering
Compton scattering (or the Compton effect) is the quantum theory of high frequency photons scattering following an interaction with a charged particle, usually an electron. Specifically, when the photon hits electrons, it releases loosely bound e ...
and synchrotron radiation
Synchrotron radiation (also known as magnetobremsstrahlung) is the electromagnetic radiation emitted when relativistic charged particles are subject to an acceleration perpendicular to their velocity (). It is produced artificially in some types ...
. A large fraction of such astronomical gamma rays are screened by Earth's atmosphere. Notable artificial sources of gamma rays include fission, such as occurs in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s, as well as high energy physics
Particle physics or high-energy physics is the study of fundamental particles and forces that constitute matter and radiation. The field also studies combinations of elementary particles up to the scale of protons and neutrons, while the stu ...
experiments, such as neutral pion decay and nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
.
A sample of gamma ray-emitting material that is used for irradiating or imaging is known as a gamma source. It is also called a radioactive source
A radioactive source is a known quantity of a radionuclide which emits ionizing radiation, typically one or more of the radiation types gamma rays, alpha particles, beta particles, and neutron radiation.
Sources can be used for irradiation, where ...
, isotope source, or radiation source, though these more general terms also apply to alpha and beta-emitting devices. Gamma sources are usually sealed to prevent radioactive contamination
Radioactive contamination, also called radiological pollution, is the deposition of, or presence of Radioactive decay, radioactive substances on surfaces or within solids, liquids, or gases (including the human body), where their presence is uni ...
, and transported in heavy shielding.
Radioactive decay (gamma decay)
Gamma rays are produced during gamma decay, which normally occurs after other forms of decay occur, such asalpha
Alpha (uppercase , lowercase ) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter ''aleph'' , whose name comes from the West Semitic word for ' ...
or beta
Beta (, ; uppercase , lowercase , or cursive ; or ) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Ancient Greek, beta represented the voiced bilabial plosive . In Modern Greek, it represe ...
decay. A radioactive nucleus can decay by the emission of an or particle. The daughter nucleus that results is usually left in an excited state. It can then decay to a lower energy state by emitting a gamma ray photon, in a process called gamma decay.
The emission of a gamma ray from an excited nucleus typically requires only 10−12 seconds. Gamma decay may also follow nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
s such as neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
, nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
, or nuclear fusion. Gamma decay is also a mode of relaxation of many excited states of atomic nuclei following other types of radioactive decay, such as beta decay, so long as these states possess the necessary component of nuclear spin. When high-energy gamma rays, electrons, or protons bombard materials, the excited atoms emit characteristic "secondary" gamma rays, which are products of the creation of excited nuclear states in the bombarded atoms. Such transitions, a form of nuclear gamma fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
, form a topic in nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies th ...
called gamma spectroscopy
Gamma-ray spectroscopy is the ''qualitative'' study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Gamma-ray spectrometry, on the other hand, is the method used to acqu ...
. Formation of fluorescent gamma rays are a rapid subtype of radioactive gamma decay.
In certain cases, the excited nuclear state that follows the emission of a beta particle or other type of excitation, may be more stable than average, and is termed a metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
excited state, if its decay takes (at least) 100 to 1000 times longer than the average 10−12 seconds. Such relatively long-lived excited nuclei are termed nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of ...
s, and their decays are termed isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 s ...
s. Such nuclei have half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
s that are more easily measurable, and rare nuclear isomers are able to stay in their excited state for minutes, hours, days, or occasionally far longer, before emitting a gamma ray. The process of isomeric transition is therefore similar to any gamma emission, but differs in that it involves the intermediate metastable excited state(s) of the nuclei. Metastable states are often characterized by high nuclear spin
Nuclear may refer to:
Physics
Relating to the nucleus of the atom:
* Nuclear engineering
* Nuclear physics
* Nuclear power
* Nuclear reactor
* Nuclear weapon
* Nuclear medicine
*Radiation therapy
*Nuclear warfare
Mathematics
* Nuclear space
* ...
, requiring a change in spin of several units or more with gamma decay, instead of a single unit transition that occurs in only 10−12 seconds. The rate of gamma decay is also slowed when the energy of excitation of the nucleus is small.
An emitted gamma ray from any type of excited state may transfer its energy directly to any electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s, but most probably to one of the K shell electrons of the atom, causing it to be ejected from that atom, in a process generally termed the photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
(external gamma rays and ultraviolet rays may also cause this effect). The photoelectric effect should not be confused with the internal conversion
Internal conversion is an atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal conversion (o ...
process, in which a gamma ray photon is not produced as an intermediate particle (rather, a "virtual gamma ray" may be thought to mediate the process).
Decay schemes
beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
emission of an electron of . Then the excited decays to the ground state (see nuclear shell model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model utilizes the Pauli exclusion principle to model the structure of atomic nuclei in terms of energy levels. The first shell model was proposed by Dmitri Ivanenk ...
) by emitting gamma rays in succession of 1.17 MeV followed by . This path is followed 99.88% of the time:
:
Another example is the alpha decay of to form ; which is followed by gamma emission. In some cases, the gamma emission spectrum of the daughter nucleus is quite simple, (e.g. /) while in other cases, such as with (/ and /), the gamma emission spectrum is complex, revealing that a series of nuclear energy levels exist.
Particle physics
Gamma rays are produced in many processes ofparticle physics
Particle physics or high-energy physics is the study of Elementary particle, fundamental particles and fundamental interaction, forces that constitute matter and radiation. The field also studies combinations of elementary particles up to the s ...
. Typically, gamma rays are the products of neutral systems which decay through electromagnetic interaction
In physics, electromagnetism is an interaction that occurs between particles with electric charge via electromagnetic fields. The electromagnetic force is one of the four fundamental forces of nature. It is the dominant force in the interacti ...
s (rather than a weak
Weak may refer to:
Songs
* Weak (AJR song), "Weak" (AJR song), 2016
* Weak (Melanie C song), "Weak" (Melanie C song), 2011
* Weak (SWV song), "Weak" (SWV song), 1993
* Weak (Skunk Anansie song), "Weak" (Skunk Anansie song), 1995
* "Weak", a son ...
or strong
Strong may refer to:
Education
* The Strong, an educational institution in Rochester, New York, United States
* Strong Hall (Lawrence, Kansas), an administrative hall of the University of Kansas
* Strong School, New Haven, Connecticut, United ...
interaction). For example, in an electron–positron annihilation
Electron–positron annihilation occurs when an electron () and a positron (, the electron's antiparticle) collide. At low energies, the result of the collision is the annihilation of the electron and positron, and the creation of energetic ph ...
, the usual products are two gamma ray photons. If the annihilating electron and positron
The positron or antielectron is the particle with an electric charge of +1''elementary charge, e'', a Spin (physics), spin of 1/2 (the same as the electron), and the same Electron rest mass, mass as an electron. It is the antiparticle (antimatt ...
are at rest, each of the resulting gamma rays has an energy of ~ 511 keV and frequency of ~ . Conversely, gamma rays above 1022 keV can interact with nuclei via pair production
Pair production is the creation of a subatomic particle and its antiparticle from a neutral boson. Examples include creating an electron and a positron, a muon and an antimuon, or a proton and an antiproton. Pair production often refers ...
of an electron and positron. Similarly, a neutral pion
In particle physics, a pion (, ) or pi meson, denoted with the Greek alphabet, Greek letter pi (letter), pi (), is any of three subatomic particles: , , and . Each pion consists of a quark and an antiquark and is therefore a meson. Pions are the ...
most often decays into two photons. Many other hadron
In particle physics, a hadron is a composite subatomic particle made of two or more quarks held together by the strong nuclear force. Pronounced , the name is derived . They are analogous to molecules, which are held together by the electri ...
s and massive boson
In particle physics, a boson ( ) is a subatomic particle whose spin quantum number has an integer value (0, 1, 2, ...). Bosons form one of the two fundamental classes of subatomic particle, the other being fermions, which have half odd-intege ...
s also decay electromagnetically. High energy physics experiments, such as the Large Hadron Collider
The Large Hadron Collider (LHC) is the world's largest and highest-energy particle accelerator. It was built by the CERN, European Organization for Nuclear Research (CERN) between 1998 and 2008, in collaboration with over 10,000 scientists, ...
, accordingly employ substantial radiation shielding. Because subatomic particle
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, lik ...
s mostly have far shorter wavelengths than atomic nuclei, particle physics gamma rays are generally several orders of magnitude more energetic than nuclear decay gamma rays. Since gamma rays are at the top of the electromagnetic spectrum in terms of energy, all extremely high-energy photons are gamma rays; for example, a photon having the Planck energy
In particle physics and physical cosmology, Planck units are a system of units of measurement defined exclusively in terms of four universal physical constants: '' c'', '' G'', '' ħ'', and ''k''B (described further below). Expressing one of ...
would be a gamma ray.
Other sources
A few gamma rays in astronomy are known to arise from gamma decay (see discussion of SN1987A), but most do not. Photons from astrophysical sources that carry energy in the gamma radiation range are often explicitly called gamma-radiation. In addition to nuclear emissions, they are often produced by sub-atomic particle and particle-photon interactions. Those include electron-positron annihilation, neutral pion decay,bremsstrahlung
In particle physics, bremsstrahlung (; ; ) is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic ...
, inverse Compton scattering
Compton scattering (or the Compton effect) is the quantum theory of high frequency photons scattering following an interaction with a charged particle, usually an electron. Specifically, when the photon hits electrons, it releases loosely bound e ...
, and synchrotron radiation
Synchrotron radiation (also known as magnetobremsstrahlung) is the electromagnetic radiation emitted when relativistic charged particles are subject to an acceleration perpendicular to their velocity (). It is produced artificially in some types ...
.
Laboratory sources
In October 2017, scientists from various European universities proposed a means for sources of GeV photons using lasers as exciters through a controlled interplay between the cascade and anomalous radiative trapping.Terrestrial thunderstorms
Thunderstorm
A thunderstorm, also known as an electrical storm or a lightning storm, is a storm characterized by the presence of lightning and its acoustics, acoustic effect on the Earth's atmosphere, known as thunder. Relatively weak thunderstorm ...
s can produce a brief pulse of gamma radiation called a terrestrial gamma-ray flash
A terrestrial gamma-ray flash (TGF), also known as dark lightning, is a burst of gamma rays produced in Earth's atmosphere. TGFs have been recorded to last 0.2 to 3.5 milliseconds, and have energies of up to 20 million electronvolts. It is spe ...
. These gamma rays are thought to be produced by high intensity static electric fields accelerating electrons, which then produce gamma rays by bremsstrahlung
In particle physics, bremsstrahlung (; ; ) is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic ...
as they collide with and are slowed by atoms in the atmosphere. Gamma rays up to 100 MeV can be emitted by terrestrial thunderstorms, and were discovered by space-borne observatories. This raises the possibility of health risks to passengers and crew on aircraft flying in or near thunderclouds.
Solar flares
The most effusivesolar flare
A solar flare is a relatively intense, localized emission of electromagnetic radiation in the Sun's atmosphere. Flares occur in active regions and are often, but not always, accompanied by coronal mass ejections, solar particle events, and ot ...
s emit across the entire EM spectrum, including γ-rays. The first confident observation occurred in 1972
Within the context of Coordinated Universal Time (UTC) it was the longest year ever, as two leap seconds were added during this 366-day year, an event which has not since been repeated. (If its start and end are defined using Solar time, ...
.
Cosmic rays
Extraterrestrial, high energy gamma rays include the gamma ray background produced when cosmic rays (either high speed electrons or protons) collide with ordinary matter, producing pair-production gamma rays at 511 keV. Alternatively,bremsstrahlung
In particle physics, bremsstrahlung (; ; ) is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic ...
are produced at energies of tens of MeV or more when cosmic ray electrons interact with nuclei of sufficiently high atomic number (see gamma ray image of the Moon near the end of this article, for illustration).

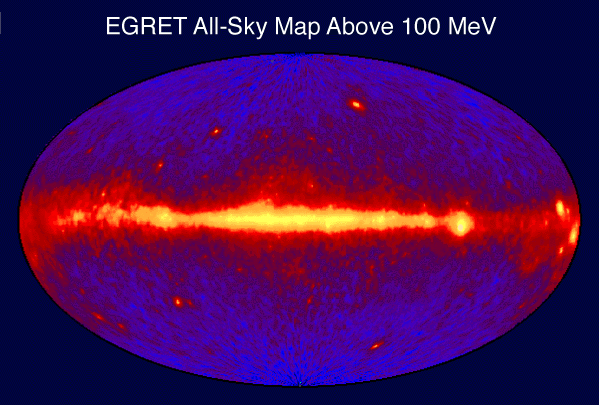
Pulsars and magnetars
The gamma ray sky (see illustration at right) is dominated by the more common and longer-term production of gamma rays that emanate frompulsar
A pulsar (''pulsating star, on the model of quasar'') is a highly magnetized rotating neutron star that emits beams of electromagnetic radiation out of its Poles of astronomical bodies#Magnetic poles, magnetic poles. This radiation can be obse ...
s within the Milky Way. Sources from the rest of the sky are mostly quasar
A quasar ( ) is an extremely Luminosity, luminous active galactic nucleus (AGN). It is sometimes known as a quasi-stellar object, abbreviated QSO. The emission from an AGN is powered by accretion onto a supermassive black hole with a mass rangi ...
s. Pulsars are thought to be neutron stars with magnetic fields that produce focused beams of radiation, and are far less energetic, more common, and much nearer sources (typically seen only in our own galaxy) than are quasars or the rarer gamma-ray burst
In gamma-ray astronomy, gamma-ray bursts (GRBs) are extremely energetic events occurring in distant Galaxy, galaxies which represent the brightest and most powerful class of explosion in the universe. These extreme Electromagnetic radiation, ele ...
sources of gamma rays. Pulsars have relatively long-lived magnetic fields that produce focused beams of relativistic speed charged particles, which emit gamma rays (bremsstrahlung) when those strike gas or dust in their nearby medium, and are decelerated. This is a similar mechanism to the production of high-energy photons in megavoltage
Megavoltage X-rays are produced by linear accelerators ("linacs") operating at voltages in excess of 1000 kV (1 MV) range, and therefore have an energy in the MeV range. The voltage in this case refers to the voltage used to accelera ...
radiation therapy
Radiation therapy or radiotherapy (RT, RTx, or XRT) is a therapy, treatment using ionizing radiation, generally provided as part of treatment of cancer, cancer therapy to either kill or control the growth of malignancy, malignant cell (biology), ...
machines (see bremsstrahlung
In particle physics, bremsstrahlung (; ; ) is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic ...
). Inverse Compton scattering, in which charged particles (usually electrons) impart energy to low-energy photons boosting them to higher energy photons. Such impacts of photons on relativistic charged particle beams is another possible mechanism of gamma ray production. Neutron stars with a very high magnetic field (magnetar
A magnetar is a type of neutron star with an extremely powerful magnetic field (~109 to 1011 T, ~1013 to 1015 G). The magnetic-field decay powers the emission of high-energy electromagnetic radiation, particularly X-rays and gamma rays.Ward; Br ...
s), thought to produce astronomical soft gamma repeater
A soft gamma repeater (SGR) is an astronomical object which emits large bursts of gamma-rays and X-rays at irregular intervals. It is conjectured that they are a type of magnetar or, alternatively, neutron stars with fossil disks around them.
H ...
s, are another relatively long-lived star-powered source of gamma radiation.
Quasars and active galaxies
More powerful gamma rays from very distantquasar
A quasar ( ) is an extremely Luminosity, luminous active galactic nucleus (AGN). It is sometimes known as a quasi-stellar object, abbreviated QSO. The emission from an AGN is powered by accretion onto a supermassive black hole with a mass rangi ...
s and closer active galaxies are thought to have a gamma ray production source similar to a particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel electric charge, charged particles to very high speeds and energies to contain them in well-defined particle beam, beams. Small accelerators are used for fundamental ...
. High energy electrons produced by the quasar, and subjected to inverse Compton scattering, synchrotron radiation
Synchrotron radiation (also known as magnetobremsstrahlung) is the electromagnetic radiation emitted when relativistic charged particles are subject to an acceleration perpendicular to their velocity (). It is produced artificially in some types ...
, or bremsstrahlung, are the likely source of the gamma rays from those objects. It is thought that a supermassive black hole
A supermassive black hole (SMBH or sometimes SBH) is the largest type of black hole, with its mass being on the order of hundreds of thousands, or millions to billions, of times the mass of the Sun (). Black holes are a class of astronomical ...
at the center of such galaxies provides the power source that intermittently destroys stars and focuses the resulting charged particles into beams that emerge from their rotational poles. When those beams interact with gas, dust, and lower energy photons they produce X-rays and gamma rays. These sources are known to fluctuate with durations of a few weeks, suggesting their relatively small size (less than a few light-weeks across). Such sources of gamma and X-rays are the most commonly visible high intensity sources outside the Milky Way galaxy. They shine not in bursts (see illustration), but relatively continuously when viewed with gamma ray telescopes. The power of a typical quasar is about 1040 watts, a small fraction of which is gamma radiation. Much of the rest is emitted as electromagnetic waves of all frequencies, including radio waves.

Gamma-ray bursts
The most intense sources of gamma rays are also the most intense sources of any type of electromagnetic radiation presently known. They are the "long duration burst" sources of gamma rays in astronomy ("long" in this context, meaning a few tens of seconds), and they are rare compared with the sources discussed above. By contrast, "short"gamma-ray burst
In gamma-ray astronomy, gamma-ray bursts (GRBs) are extremely energetic events occurring in distant Galaxy, galaxies which represent the brightest and most powerful class of explosion in the universe. These extreme Electromagnetic radiation, ele ...
s of two seconds or less, which are not associated with supernovae, are thought to produce gamma rays during the collision of pairs of neutron stars, or a neutron star and a black hole
A black hole is a massive, compact astronomical object so dense that its gravity prevents anything from escaping, even light. Albert Einstein's theory of general relativity predicts that a sufficiently compact mass will form a black hole. Th ...
.
The so-called ''long-duration'' gamma-ray bursts produce a total energy output of about 1044 joules (as much energy as the Sun
The Sun is the star at the centre of the Solar System. It is a massive, nearly perfect sphere of hot plasma, heated to incandescence by nuclear fusion reactions in its core, radiating the energy from its surface mainly as visible light a ...
will produce in its entire life-time) but in a period of only 20 to 40 seconds. Gamma rays are approximately 50% of the total energy output. The leading hypotheses for the mechanism of production of these highest-known intensity beams of radiation, are inverse Compton scattering
Compton scattering (or the Compton effect) is the quantum theory of high frequency photons scattering following an interaction with a charged particle, usually an electron. Specifically, when the photon hits electrons, it releases loosely bound e ...
and synchrotron radiation
Synchrotron radiation (also known as magnetobremsstrahlung) is the electromagnetic radiation emitted when relativistic charged particles are subject to an acceleration perpendicular to their velocity (). It is produced artificially in some types ...
from high-energy charged particles. These processes occur as relativistic charged particles leave the region of the event horizon of a newly formed black hole
A black hole is a massive, compact astronomical object so dense that its gravity prevents anything from escaping, even light. Albert Einstein's theory of general relativity predicts that a sufficiently compact mass will form a black hole. Th ...
created during supernova explosion. The beam of particles moving at relativistic speeds are focused for a few tens of seconds by the magnetic field of the exploding hypernova
A hypernova is a very energetic supernova which is believed to result from an extreme core collapse scenario. In this case, a massive star (>30 solar masses) collapses to form a rotating black hole emitting twin astrophysical jets and surrounded b ...
. The fusion explosion of the hypernova drives the energetics of the process. If the narrowly directed beam happens to be pointed toward the Earth, it shines at gamma ray frequencies with such intensity, that it can be detected even at distances of up to 10 billion light years, which is close to the edge of the visible universe.
Properties
Penetration of matter
alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s, which can be stopped by paper or skin, and beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and � ...
s, which can be shielded by thin aluminium. Gamma rays are best absorbed by materials with high atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
s (''Z'') and high density, which contribute to the total stopping power. Because of this, a lead (high ''Z'') shield is 20–30% better as a gamma shield than an equal mass of a low-''Z'' shielding material, such as aluminium, concrete, water, or soil; lead's major advantage is not in lower weight, but rather its compactness due to its higher density. Protective clothing, goggles and respirators can protect from internal contact with or ingestion of alpha or beta emitting particles, but provide no protection from gamma radiation from external sources.
The higher the energy of the gamma rays, the thicker the shielding made from the same shielding material is required. Materials for shielding gamma rays are typically measured by the thickness required to reduce the intensity of the gamma rays by one half (the half-value layer or HVL). For example, gamma rays that require 1 cm (0.4 inch) of lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
to reduce their intensity by 50% will also have their intensity reduced in half by of granite
Granite ( ) is a coarse-grained (phanerite, phaneritic) intrusive rock, intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly coo ...
rock, 6 cm (2.5 inches) of concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactur ...
, or 9 cm (3.5 inches) of packed soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
. However, the mass of this much concrete or soil is only 20–30% greater than that of lead with the same absorption capability.
Depleted uranium
Depleted uranium (DU), also referred to in the past as Q-metal, depletalloy, or D-38, is uranium with a lower content of the fissile isotope Uranium-235, 235U than natural uranium. The less radioactive and non-fissile Uranium-238, 238U is the m ...
is sometimes used for shielding in portable gamma ray sources, due to the smaller half-value layer when compared to lead (around 0.6 times the thickness for common gamma ray sources, i.e. Iridium-192 and Cobalt-60) and cheaper cost compared to tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
.
In a nuclear power plant, shielding can be provided by steel and concrete in the pressure and particle containment vessel, while water provides a radiation shielding of fuel rods during storage or transport into the reactor core. The loss of water or removal of a "hot" fuel assembly into the air would result in much higher radiation levels than when kept under water.
Matter interaction
photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
: This describes the case in which a gamma photon interacts with and transfers its energy to an atomic electron, causing the ejection of that electron from the atom. The kinetic energy of the resulting photoelectron
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
is equal to the energy of the incident gamma photon minus the energy that originally bound the electron to the atom (binding energy). The photoelectric effect is the dominant energy transfer mechanism for X-ray and gamma ray photons with energies below 50 keV (thousand electronvolts), but it is much less important at higher energies.
* Compton scattering
Compton scattering (or the Compton effect) is the quantum theory of high frequency photons scattering following an interaction with a charged particle, usually an electron. Specifically, when the photon hits electrons, it releases loosely bound e ...
: This is an interaction in which an incident gamma photon loses enough energy to an atomic electron to cause its ejection, with the remainder of the original photon's energy emitted as a new, lower energy gamma photon whose emission direction is different from that of the incident gamma photon, hence the term "scattering". The probability of Compton scattering decreases with increasing photon energy. It is thought to be the principal absorption mechanism for gamma rays in the intermediate energy range 100 keV to 10 MeV. It is relatively independent of the atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
of the absorbing material, which is why very dense materials like lead are only modestly better shields, on a ''per weight'' basis, than are less dense materials.
* Pair production
Pair production is the creation of a subatomic particle and its antiparticle from a neutral boson. Examples include creating an electron and a positron, a muon and an antimuon, or a proton and an antiproton. Pair production often refers ...
: This becomes possible with gamma energies exceeding 1.02 MeV, and becomes important as an absorption mechanism at energies over 5 MeV (see illustration at right, for lead). By interaction with the electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
of a nucleus, the energy of the incident photon is converted into the mass of an electron-positron pair. Any gamma energy in excess of the equivalent rest mass of the two particles (totaling at least 1.02 MeV) appears as the kinetic energy of the pair and in the recoil of the emitting nucleus. At the end of the positron's range
Range may refer to:
Geography
* Range (geographic), a chain of hills or mountains; a somewhat linear, complex mountainous or hilly area (cordillera, sierra)
** Mountain range, a group of mountains bordered by lowlands
* Range, a term used to i ...
, it combines with a free electron, and the two annihilate, and the entire mass of these two is then converted into two gamma photons of at least 0.51 MeV energy each (or higher according to the kinetic energy of the annihilated particles).
The secondary electrons (and/or positrons) produced in any of these three processes frequently have enough energy to produce much ionization
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged at ...
themselves.
Additionally, gamma rays, particularly high energy ones, can interact with atomic nuclei resulting in ejection of particles in photodisintegration
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The ...
, or in some cases, even nuclear fission ( photofission).
Light interaction
High-energy (from 80 GeV to ~10 TeV) gamma rays arriving from far-distant quasars are used to estimate theextragalactic background light
The diffuse extragalactic background light (EBL) is all the accumulated radiation in the universe due to star formation processes, plus a contribution from active galactic nuclei (AGNs). This radiation covers almost all wavelengths of the electrom ...
in the universe: The highest-energy rays interact more readily with the background light photons and thus the density of the background light may be estimated by analyzing the incoming gamma ray spectra.
Gamma spectroscopy
Gamma spectroscopy is the study of the energetic transitions in atomic nuclei, which are generally associated with the absorption or emission of gamma rays. As in opticalspectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectro ...
(see Franck–Condon effect) the absorption of gamma rays by a nucleus is especially likely (i.e., peaks in a "resonance") when the energy of the gamma ray is the same as that of an energy transition in the nucleus. In the case of gamma rays, such a resonance is seen in the technique of Mössbauer spectroscopy
Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer (sometimes written "Moessbauer", German: "Mößbauer") in 1958, consists of the nearly recoil-free emission and a ...
. In the Mössbauer effect the narrow resonance absorption for nuclear gamma absorption can be successfully attained by physically immobilizing atomic nuclei in a crystal. The immobilization of nuclei at both ends of a gamma resonance interaction is required so that no gamma energy is lost to the kinetic energy of recoiling nuclei at either the emitting or absorbing end of a gamma transition. Such loss of energy causes gamma ray resonance absorption to fail. However, when emitted gamma rays carry essentially all of the energy of the atomic nuclear de-excitation that produces them, this energy is also sufficient to excite the same energy state in a second immobilized nucleus of the same type.
Applications
topaz
Topaz is a silicate mineral made of aluminium, aluminum and fluorine with the chemical formula aluminium, Alsilicon, Sioxygen, O(fluorine, F, hydroxide, OH). It is used as a gemstone in jewelry and other adornments. Common topaz in its natural ...
into blue topaz.
Non-contact industrial sensors commonly use sources of gamma radiation in refining, mining, chemicals, food, soaps and detergents, and pulp and paper industries, for the measurement of levels, density, and thicknesses. Gamma-ray sensors are also used for measuring the fluid levels in water and oil industries. Typically, these use Co-60 or Cs-137 isotopes as the radiation source.
In the US, gamma ray detectors are beginning to be used as part of the Container Security Initiative (CSI). These machines are advertised to be able to scan 30 containers per hour.
Gamma radiation is often used to kill living organisms, in a process called irradiation
Irradiation is the process by which an object is exposed to radiation. An irradiator is a device used to expose an object to radiation, most often gamma radiation, for a variety of purposes. Irradiators may be used for sterilizing medical and p ...
. Applications of this include the sterilization of medical equipment (as an alternative to autoclave
An autoclave is a machine used to carry out industrial and scientific processes requiring elevated temperature and pressure in relation to ambient pressure and/or temperature. Autoclaves are used before surgical procedures to perform steriliza ...
s or chemical means), the removal of decay-causing bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
from many foods and the prevention of the sprouting of fruit and vegetables to maintain freshness and flavor.
Despite their cancer-causing properties, gamma rays are also used to treat some types of cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
, since the rays also kill cancer cells. In the procedure called gamma-knife surgery, multiple concentrated beams of gamma rays are directed to the growth in order to kill the cancerous cells. The beams are aimed from different angles to concentrate the radiation on the growth while minimizing damage to surrounding tissues.
Gamma rays are also used for diagnostic purposes in nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
in imaging techniques. A number of different gamma-emitting radioisotopes are used. For example, in a PET scan
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in Metabolism, metabolic processes, and in other physiological activities including bloo ...
a radiolabeled sugar called fluorodeoxyglucose emits positron
The positron or antielectron is the particle with an electric charge of +1''elementary charge, e'', a Spin (physics), spin of 1/2 (the same as the electron), and the same Electron rest mass, mass as an electron. It is the antiparticle (antimatt ...
s that are annihilated by electrons, producing pairs of gamma rays that highlight cancer as the cancer often has a higher metabolic rate than the surrounding tissues. The most common gamma emitter used in medical applications is the nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of ...
technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used Radiophar ...
which emits gamma rays in the same energy range as diagnostic X-rays. When this radionuclide tracer is administered to a patient, a gamma camera
A gamma camera (γ-camera), also called a scintillation camera or Anger camera, is a device used to image gamma radiation emitting radioisotopes, a technique known as scintigraphy. The applications of scintigraphy include early drug development ...
can be used to form an image of the radioisotope's distribution by detecting the gamma radiation emitted (see also SPECT
Single-photon emission computed tomography (SPECT, or less commonly, SPET) is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera (that is, ...
). Depending on which molecule has been labeled with the tracer, such techniques can be employed to diagnose a wide range of conditions (for example, the spread of cancer to the bones via bone scan
A bone scan or bone scintigraphy is a nuclear medicine imaging technique used to help diagnose and assess different bone diseases. These include cancer of the bone or metastasis, location of bone inflammation and fractures (that may not be vis ...
).
Health effects
Gamma rays cause damage at a cellular level and are penetrating, causing diffuse damage throughout the body. However, they are less ionising than alpha or beta particles, which are less penetrating. Low levels of gamma rays cause astochastic Stochastic (; ) is the property of being well-described by a random probability distribution. ''Stochasticity'' and ''randomness'' are technically distinct concepts: the former refers to a modeling approach, while the latter describes phenomena; i ...
health risk, which for radiation dose assessment is defined as the ''probability'' of cancer induction and genetic damage. The International Commission on Radiological Protection
The International Commission on Radiological Protection (ICRP) is an independent, international, non-governmental organization, with the mission to protect people, animals, and the environment from the harmful effects of ionising radiation. Its ...
says "In the low dose range, below about 100 mSv, it is scientifically plausible to assume that the incidence of cancer or heritable effects will rise in direct proportion to an increase in the equivalent dose in the relevant organs and tissues" (The unit mSv is milli- Seivert). High doses produce deterministic
Determinism is the metaphysical view that all events within the universe (or multiverse) can occur only in one possible way. Deterministic theories throughout the history of philosophy have developed from diverse and sometimes overlapping mo ...
effects, which is the ''severity'' of acute tissue damage that is certain to happen. These effects are compared to the physical quantity absorbed dose
Absorbed dose is a dose quantity which represents the specific energy (energy per unit mass) deposited by ionizing radiation in living matter. Absorbed dose is used in the calculation of dose uptake in living tissue in both radiation protecti ...
measured by the unit gray
Grey (more frequent in British English) or gray (more frequent in American English) is an intermediate color between black and white. It is a neutral or achromatic color, meaning that it has no chroma. It is the color of a cloud-covered s ...
(Gy).
Effects and body response
When gamma radiation breaks DNA molecules, a cell may be able to repair the damaged genetic material, within limits. However, a study of Rothkamm and Lobrich concerning X-ray radiation has shown that this repair process works well after high-dose exposure but is much slower in the case of a low-dose exposure. Studies have shown low-dose gamma radiation may be enough to cause cancer. In a study of mice, they were given human-relevant low-dose gamma radiation, with genotoxic effects 45 days after continuous low-dose gamma radiation, with significant increases of chromosomal damage, DNA lesions and phenotypic mutations in blood cells of irradiated animals, covering the three types of genotoxic activity. Another study studied the effects of acute ionizing gamma radiation in rats, up to 10 Gy, and who ended up showing acute oxidative protein damage, DNA damage, cardiac troponin T carbonylation, and long-termcardiomyopathy
Cardiomyopathy is a group of primary diseases of the heart muscle. Early on there may be few or no symptoms. As the disease worsens, shortness of breath, feeling tired, and swelling of the legs may occur, due to the onset of heart failure. A ...
.
Risk assessment
Natural gamma radiation in the United Kingdom of accounts for about 13% of the average radiation dose. Natural exposure to gamma rays is about 1 to 2 mSv per year, and the average total amount of radiation received in one year per inhabitant in the USA is 3.6 mSv. There might be a small increase in the dose around small particles ofdepleted uranium
Depleted uranium (DU), also referred to in the past as Q-metal, depletalloy, or D-38, is uranium with a lower content of the fissile isotope Uranium-235, 235U than natural uranium. The less radioactive and non-fissile Uranium-238, 238U is the m ...
should these enter the human body from spent munitions, caused by increased effects of natural gamma radiation.
By comparison, the radiation dose from chest radiography
Radiography is an imaging technology, imaging technique using X-rays, gamma rays, or similar ionizing radiation and non-ionizing radiation to view the internal form of an object. Applications of radiography include medical ("diagnostic" radiog ...
(about 0.06 mSv) is a fraction of the annual naturally occurring background radiation dose. A chest CT delivers 5 to 8 mSv. A whole-body PET
A pet, or companion animal, is an animal kept primarily for a person's company or entertainment rather than as a working animal, livestock, or a laboratory animal. Popular pets are often considered to have attractive/ cute appearances, inte ...
/CT scan can deliver 14 to 32 mSv depending on the protocol. The dose from fluoroscopy
Fluoroscopy (), informally referred to as "fluoro", is an imaging technique that uses X-rays to obtain real-time moving images of the interior of an object. In its primary application of medical imaging, a fluoroscope () allows a surgeon to see t ...
of the stomach is much higher, approximately 50 mSv (14 times the annual background).
An acute full-body equivalent single exposure dose of 1 Sv (1000 mSv), or 1 Gy, will cause mild symptoms of acute radiation sickness, such as nausea and vomiting; and a dose of 2.0–3.5 Sv (2.0–3.5 Gy) causes more severe symptoms (i.e. nausea, diarrhea, hair loss, hemorrhaging
Bleeding, hemorrhage, haemorrhage or blood loss, is blood escaping from the circulatory system from damaged blood vessels. Bleeding can occur internally, or externally either through a natural opening such as the mouth, nose, ear, urethra, ...
, and inability to fight infections), and will cause death in a sizable number of cases—about 10% to 35% without medical treatment. A dose of 3–5 Sv (3–5 Gy) is considered approximately the LD50 (or the lethal dose for 50% of exposed population) for an acute exposure to radiation even with standard medical treatment. A dose higher than 5 Sv (5 Gy) brings an increasing chance of death above 50%. Above 7.5–10 Sv (7.5–10 Gy) to the entire body, even extraordinary treatment, such as bone-marrow transplants, will not prevent the death of the individual exposed (see radiation poisoning). (Doses much larger than this may, however, be delivered to selected parts of the body in the course of radiation therapy
Radiation therapy or radiotherapy (RT, RTx, or XRT) is a therapy, treatment using ionizing radiation, generally provided as part of treatment of cancer, cancer therapy to either kill or control the growth of malignancy, malignant cell (biology), ...
.)
For low-dose exposure, for example among nuclear workers, who receive an average yearly radiation dose of 19 mSv, the risk of dying from cancer (excluding leukemia
Leukemia ( also spelled leukaemia; pronounced ) is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or '' ...
) increases by 2 percent. For a dose of 100 mSv, the risk increase is 10 percent. By comparison, risk of dying from cancer was increased by 32 percent for the survivors of the atomic bombing of Hiroshima and Nagasaki.
Units of measurement and exposure
The following table shows radiation quantities in SI and non-SI units: The measure of the ionizing effect of gamma and X-rays in dry air is called the exposure, for which a legacy unit, the röntgen, was used from 1928. This has been replaced bykerma
Kerma was the capital city of the Kerma culture, which was founded in present-day Sudan before 3500 BC. Kerma is one of the largest archaeological sites in ancient Nubia. It has produced decades of extensive excavations and research, including t ...
, now mainly used for instrument calibration purposes but not for received dose effect. The effect of gamma and other ionizing radiation on living tissue is more closely related to the amount of energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
deposited in tissue rather than the ionisation of air, and replacement radiometric units and quantities for radiation protection
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposu ...
have been defined and developed from 1953 onwards. These are:
* The gray
Grey (more frequent in British English) or gray (more frequent in American English) is an intermediate color between black and white. It is a neutral or achromatic color, meaning that it has no chroma. It is the color of a cloud-covered s ...
(Gy), is the SI unit of absorbed dose
Absorbed dose is a dose quantity which represents the specific energy (energy per unit mass) deposited by ionizing radiation in living matter. Absorbed dose is used in the calculation of dose uptake in living tissue in both radiation protecti ...
, which is the amount of radiation energy deposited in the irradiated material. For gamma radiation this is numerically equivalent to equivalent dose
Equivalent dose (symbol ''H'') is a dose quantity representing the stochastic health effects of low levels of ionizing radiation on the human body which represents the probability of radiation-induced cancer and genetic damage. It is derived fro ...
measured by the sievert
The sievert (symbol: SvPlease note there are two non-SI units that use the same Sv abbreviation: the sverdrup and svedberg.) is a derived unit in the International System of Units (SI) intended to represent the stochastic health risk of ionizin ...
, which indicates the stochastic biological effect of low levels of radiation on human tissue. The radiation weighting conversion factor from absorbed dose to equivalent dose is 1 for gamma, whereas alpha particles have a factor of 20, reflecting their greater ionising effect on tissue.
* The rad is the deprecated CGS unit for absorbed dose and the rem is the deprecated CGS unit of equivalent dose, used mainly in the USA.
Distinction from X-rays
 The conventional distinction between X-rays and gamma rays has changed over time. Originally, the electromagnetic radiation emitted by
The conventional distinction between X-rays and gamma rays has changed over time. Originally, the electromagnetic radiation emitted by X-ray tube
An X-ray tube is a vacuum tube that converts electrical input power into X-rays. The availability of this controllable source of X-rays created the field of radiography, the imaging of partly opaque objects with penetrating radiation. In contras ...
s almost invariably had a longer wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
than the radiation (gamma rays) emitted by radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
nuclei. Older literature distinguished between X- and gamma radiation on the basis of wavelength, with radiation shorter than some arbitrary wavelength, such as 10−11 m, defined as gamma rays. Since the energy of photons is proportional to their frequency and inversely proportional to wavelength, this past distinction between X-rays and gamma rays can also be thought of in terms of its energy, with gamma rays considered to be higher energy electromagnetic radiation than are X-rays.
However, since current artificial sources are now able to duplicate any electromagnetic radiation that originates in the nucleus, as well as far higher energies, the wavelengths characteristic of radioactive gamma ray sources vs. other types now completely overlap. Thus, gamma rays are now usually distinguished by their origin: X-rays are emitted by definition by electrons outside the nucleus, while gamma rays are emitted by the nucleus. Exceptions to this convention occur in astronomy, where gamma decay is seen in the afterglow of certain supernovas, but radiation from high energy processes known to involve other radiation sources than radioactive decay is still classed as gamma radiation.
For example, modern high-energy X-rays produced by linear accelerators for megavoltage
Megavoltage X-rays are produced by linear accelerators ("linacs") operating at voltages in excess of 1000 kV (1 MV) range, and therefore have an energy in the MeV range. The voltage in this case refers to the voltage used to accelera ...
treatment in cancer often have higher energy (4 to 25 MeV) than do most classical gamma rays produced by nuclear gamma decay
Gamma (; uppercase , lowercase ; ) is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter normally repr ...
. One of the most common gamma ray emitting isotopes used in diagnostic nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
, technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used Radiophar ...
, produces gamma radiation of the same energy (140 keV) as that produced by diagnostic X-ray machines, but of significantly lower energy than therapeutic photons from linear particle accelerators. In the medical community today, the convention that radiation produced by nuclear decay is the only type referred to as "gamma" radiation is still respected.
Due to this broad overlap in energy ranges, in physics the two types of electromagnetic radiation are now often defined by their origin: X-rays are emitted by electrons (either in orbitals outside of the nucleus, or while being accelerated to produce bremsstrahlung
In particle physics, bremsstrahlung (; ; ) is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic ...
-type radiation), while gamma rays are emitted by the nucleus or by means of other particle decay
In particle physics, particle decay is the spontaneous process of one unstable subatomic particle transforming into multiple other particles. The particles created in this process (the ''final state'') must each be less massive than the original ...
s or annihilation events. There is no lower limit to the energy of photons produced by nuclear reactions, and thus ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
or lower energy photons produced by these processes would also be defined as "gamma rays" (indeed, this happens for the isomeric transition of the extremely low-energy isomer 229mTh). The only naming-convention that is still universally respected is the rule that electromagnetic radiation that is known to be of atomic nuclear origin is ''always'' referred to as "gamma rays", and never as X-rays. However, in physics and astronomy, the converse convention (that all gamma rays are considered to be of nuclear origin) is frequently violated.
In astronomy, higher energy gamma and X-rays are defined by energy, since the processes that produce them may be uncertain and photon energy, not origin, determines the required astronomical detectors needed. High-energy photons occur in nature that are known to be produced by processes other than nuclear decay but are still referred to as gamma radiation. An example is "gamma rays" from lightning discharges at 10 to 20 MeV, and known to be produced by the bremsstrahlung mechanism.
Another example is gamma-ray bursts, now known to be produced from processes too powerful to involve simple collections of atoms undergoing radioactive decay. This is part and parcel of the general realization that many gamma rays produced in astronomical processes result not from radioactive decay or particle annihilation, but rather in non-radioactive processes similar to X-rays. Although the gamma rays of astronomy often come from non-radioactive events, a few gamma rays in astronomy are specifically known to originate from gamma decay of nuclei (as demonstrated by their spectra and emission half life). A classic example is that of supernova SN 1987A
SN 1987A was a Type II supernova in the Large Magellanic Cloud, a dwarf satellite galaxy of the Milky Way. It occurred approximately from Earth and was the closest observed supernova since Kepler's Supernova in 1604. Light and neutrinos ...
, which emits an "afterglow" of gamma-ray photons from the decay of newly made radioactive nickel-56 and cobalt-56. Most gamma rays in astronomy, however, arise by other mechanisms.
See also
*Annihilation
In particle physics, annihilation is the process that occurs when a subatomic particle collides with its respective antiparticle to produce other particles, such as an electron colliding with a positron to produce two photons. The total energy a ...
* Galactic Center GeV excess
The Galactic Center GeV Excess (GCE) is an unexpected surplus of gamma-ray radiation in the center of the Milky Way
The Milky Way or Milky Way Galaxy is the galaxy that includes the Solar System, with the name describing the #Appearance ...
* Gaseous ionization detectors
* Very-high-energy gamma ray
A very-high-energy gamma ray (VHEGR) is Gamma ray, gamma radiation with photon energy, photon energies of 100 GeV (gigaelectronvolt) to 100 TeV (teraelectronvolt), i.e., 1011 to 1014 electronvolts. This is approximately equal to
wavelengths betwe ...
* Ultra-high-energy gamma ray
Explanatory notes
References
External links
Basic reference on several types of radiation
Radiation Q & A
Radiation information
The Lund/LBNL Nuclear Data Search
– Contains information on gamma-ray energies from isotopes.
The LIVEChart of Nuclides – IAEA
with filter on gamma-ray energy
Health Physics Society Public Education Website
{{Authority control Articles containing video clips Electromagnetic spectrum IARC Group 1 carcinogens Nuclear physics Radiation Radioactivity