free radical polymerization on:
[Wikipedia]
[Google]
[Amazon]
In
 ; Photolysis: Radiation cleaves a bond homolytically, producing two radicals (Figure 2). This method is used most often with metal iodides, metal alkyls, and azo compounds.
; Photolysis: Radiation cleaves a bond homolytically, producing two radicals (Figure 2). This method is used most often with metal iodides, metal alkyls, and azo compounds.  Photoinitiation can also occur by bi-molecular H abstraction when the radical is in its lowest triplet excited state. An acceptable photoinitiator system should fulfill the following requirements:
;* High absorptivity in the 300–400 nm range.
;* Efficient generation of radicals capable of attacking the
Photoinitiation can also occur by bi-molecular H abstraction when the radical is in its lowest triplet excited state. An acceptable photoinitiator system should fulfill the following requirements:
;* High absorptivity in the 300–400 nm range.
;* Efficient generation of radicals capable of attacking the  ; Ionizing radiation: α-, β-, γ-, or
; Ionizing radiation: α-, β-, γ-, or  ;
; ; Plasma: A gaseous monomer is placed in an electric discharge at low pressures under conditions where a plasma (ionized gaseous molecules) is created. In some cases, the system is heated and/or placed in a
; Plasma: A gaseous monomer is placed in an electric discharge at low pressures under conditions where a plasma (ionized gaseous molecules) is created. In some cases, the system is heated and/or placed in a  This type of initiating system contains a metallocene, an initiator, and a heteroaromatic diketo
This type of initiating system contains a metallocene, an initiator, and a heteroaromatic diketo
 ;Other recombination pathways: Two radical initiators recombine before initiating a chain, but not in the solvent cage (Figure 9).
;Other recombination pathways: Two radical initiators recombine before initiating a chain, but not in the solvent cage (Figure 9).
 ;Side reactions: One radical is produced instead of the three radicals that could be produced (Figure 10).
;Side reactions: One radical is produced instead of the three radicals that could be produced (Figure 10).

 Once a chain has been initiated, the chain propagates (Figure 13) until there are no more monomers (
Once a chain has been initiated, the chain propagates (Figure 13) until there are no more monomers (
 ** '' Radical disproportionation:'' a hydrogen atom from one chain end is abstracted to another, producing a polymer with a terminal unsaturated group and a polymer with a terminal saturated group (Figure 15).
** '' Radical disproportionation:'' a hydrogen atom from one chain end is abstracted to another, producing a polymer with a terminal unsaturated group and a polymer with a terminal saturated group (Figure 15). 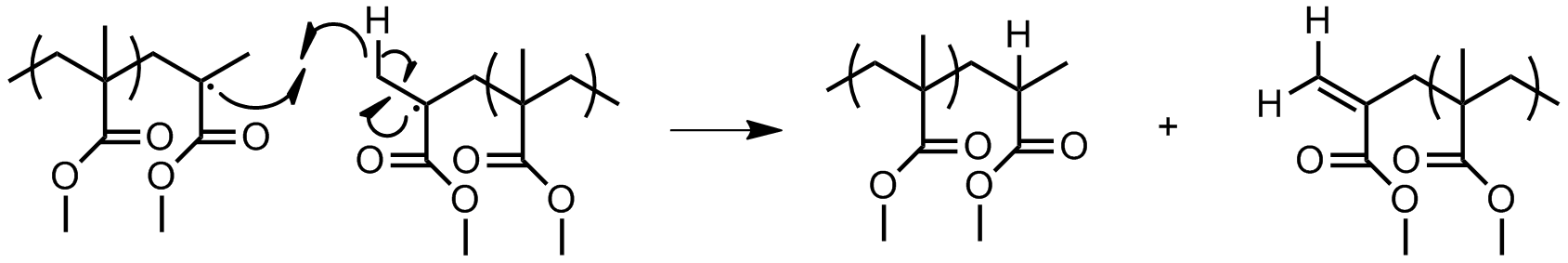 * Combination of an active chain end with an initiator radical (Figure 16).
* Combination of an active chain end with an initiator radical (Figure 16).  * Interaction with impurities or inhibitors.
* Interaction with impurities or inhibitors. 

 The effectiveness of chain transfer involving solvent molecules depends on the amount of solvent present (more solvent leads to greater probability of transfer), the strength of the bond involved in the abstraction step (weaker bond leads to greater probability of transfer), and the stability of the solvent radical that is formed (greater stability leads to greater probability of transfer). Halogens, except fluorine, are easily transferred.
* ''To monomer:'' a hydrogen atom is abstracted from a monomer. While this does create a radical on the affected monomer, resonance stabilization of this radical discourages further propagation (Figure 20).
The effectiveness of chain transfer involving solvent molecules depends on the amount of solvent present (more solvent leads to greater probability of transfer), the strength of the bond involved in the abstraction step (weaker bond leads to greater probability of transfer), and the stability of the solvent radical that is formed (greater stability leads to greater probability of transfer). Halogens, except fluorine, are easily transferred.
* ''To monomer:'' a hydrogen atom is abstracted from a monomer. While this does create a radical on the affected monomer, resonance stabilization of this radical discourages further propagation (Figure 20).  * ''To initiator:'' a polymer chain reacts with an initiator, which terminates that polymer chain, but creates a new radical initiator (Figure 21). This initiator can then begin new polymer chains. Therefore, contrary to the other forms of chain transfer, chain transfer to the initiator does allow for further propagation. Peroxide initiators are especially sensitive to chain transfer.
* ''To initiator:'' a polymer chain reacts with an initiator, which terminates that polymer chain, but creates a new radical initiator (Figure 21). This initiator can then begin new polymer chains. Therefore, contrary to the other forms of chain transfer, chain transfer to the initiator does allow for further propagation. Peroxide initiators are especially sensitive to chain transfer.  * ''To polymer:'' the radical of a polymer chain abstracts a hydrogen atom from somewhere on another polymer chain (Figure 22). This terminates the growth of one polymer chain, but allows the other to branch and resume growing. This reaction step changes neither the number of polymer chains nor the number of monomers which have been polymerized, so that the number-average
* ''To polymer:'' the radical of a polymer chain abstracts a hydrogen atom from somewhere on another polymer chain (Figure 22). This terminates the growth of one polymer chain, but allows the other to branch and resume growing. This reaction step changes neither the number of polymer chains nor the number of monomers which have been polymerized, so that the number-average  ''Effects of chain transfer:'' The most obvious effect of chain transfer is a decrease in the polymer chain length. If the rate of transfer is much larger than the rate of propagation, then very small polymers are formed with chain lengths of 2-5 repeating units (
''Effects of chain transfer:'' The most obvious effect of chain transfer is a decrease in the polymer chain length. If the rate of transfer is much larger than the rate of propagation, then very small polymers are formed with chain lengths of 2-5 repeating units (

 Because the chain end is functionalized with the
Because the chain end is functionalized with the
polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are ...
, free-radical polymerization (FRP) is a method of polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
by which a polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
forms by the successive addition of free-radical building blocks ( repeat units). Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
units, thereby growing the polymer chain.
Free-radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of free-radical chemical interactions makes this one of the most versatile forms of polymerization available and allows facile reactions of polymeric free-radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the United States were produced by free-radical polymerization.
Free-radical polymerization is a type of chain-growth polymerization, along with anionic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
, cationic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
and coordination polymerization.
Initiation
Initiation is the first step of thepolymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
process. During initiation, an active center is created from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators. Radical initiation works best on the carbon–carbon double bond of vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from viny ...
monomers and the carbon–oxygen double bond in aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
and ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
. Initiation has two steps. In the first step, one or two radicals
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
are created from the initiating molecules. In the second step, radicals are transferred from the initiator molecules to the monomer units present. Several choices are available for these initiators.
Types of initiation and the initiators
;Thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
: The initiator is heated until a bond is homolytically cleaved, producing two radicals (Figure 1). This method is used most often with organic peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
s or azo compounds.  ; Photolysis: Radiation cleaves a bond homolytically, producing two radicals (Figure 2). This method is used most often with metal iodides, metal alkyls, and azo compounds.
; Photolysis: Radiation cleaves a bond homolytically, producing two radicals (Figure 2). This method is used most often with metal iodides, metal alkyls, and azo compounds.  Photoinitiation can also occur by bi-molecular H abstraction when the radical is in its lowest triplet excited state. An acceptable photoinitiator system should fulfill the following requirements:
;* High absorptivity in the 300–400 nm range.
;* Efficient generation of radicals capable of attacking the
Photoinitiation can also occur by bi-molecular H abstraction when the radical is in its lowest triplet excited state. An acceptable photoinitiator system should fulfill the following requirements:
;* High absorptivity in the 300–400 nm range.
;* Efficient generation of radicals capable of attacking the alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
double bond of vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from viny ...
monomers.
;* Adequate solubility in the binder system ( prepolymer + monomer).
;* Should not impart yellowing or unpleasant odors to the cured material.
;* The photoinitiator and any byproducts resulting from its use should be non-toxic.
;Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
reactions: Reduction of hydrogen peroxide or an alkyl hydrogen peroxide by iron (Figure 3). Other reductants such as Cr2+, V2+, Ti3+, Co2+, and Cu+ can be employed in place of ferrous ion in many instances.
; Persulfates: The dissociation of a persulfate in the aqueous phase (Figure 4). This method is useful in emulsion polymerizations, in which the radical diffuses into a hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, ...
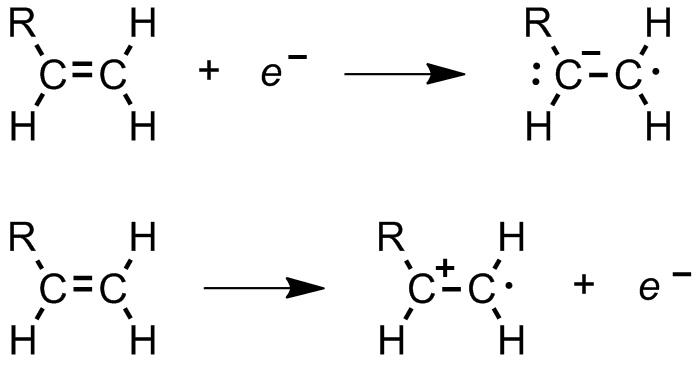
monomer-containing droplet.  ; Ionizing radiation: α-, β-, γ-, or
; Ionizing radiation: α-, β-, γ-, or x-ray
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ' ...
s cause ejection of an electron from the initiating species, followed by dissociation and electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. T ...
to produce a radical (Figure 5).  ;
;Electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
: Electrolysis of a solution containing both monomer and electrolyte. A monomer molecule will receive an electron at the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction i ...
to become a radical anion, and a monomer molecule will give up an electron at the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemoni ...
to form a radical cation (Figure 6). The radical ions then initiate free radical (and/or ionic) polymerization. This type of initiation is especially useful for coating metal surfaces with polymer films.  ; Plasma: A gaseous monomer is placed in an electric discharge at low pressures under conditions where a plasma (ionized gaseous molecules) is created. In some cases, the system is heated and/or placed in a
; Plasma: A gaseous monomer is placed in an electric discharge at low pressures under conditions where a plasma (ionized gaseous molecules) is created. In some cases, the system is heated and/or placed in a radiofrequency
Radio frequency (RF) is the oscillation rate of an alternating electric current or voltage or of a magnetic, electric or electromagnetic field or mechanical system in the frequency range from around to around . This is roughly between the upper ...
field to assist in creating the plasma.
;Sonication
A sonicator at the Weizmann Institute of Science during sonicationSonication is the act of applying sound energy to agitate particles in a sample, for various purposes such as the extraction of multiple compounds from plants, microalgae and seawe ...
: High-intensity ultrasound at frequencies beyond the range of human hearing (16 kHz) can be applied to a monomer. Initiation results from the effects of cavitation
Cavitation is a phenomenon in which the static pressure of a liquid reduces to below the liquid's vapour pressure, leading to the formation of small vapor-filled cavities in the liquid. When subjected to higher pressure, these cavities, cal ...
(the formation and collapse of cavities in the liquid). The collapse of the cavities generates very high local temperatures and pressures. This results in the formation of excited electronic states, which in turn lead to bond breakage and radical formation.
;Ternary initiators: A ternary
Ternary (from Latin ''ternarius'') or trinary is an adjective meaning "composed of three items". It can refer to:
Mathematics and logic
* Ternary numeral system, a base-3 counting system
** Balanced ternary, a positional numeral system, usef ...
initiator is the combination of several types of initiators into one initiating system. The types of initiators are chosen based on the properties they are known to induce in the polymers they produce. For example, poly(methyl methacrylate) has been synthesized by the ternary system benzoyl peroxide-3,6-bis(''o''-carboxybenzoyl)-''N''-isopropylcarbazole-di-η5-indenylzicronium dichloride (Figure 7).  This type of initiating system contains a metallocene, an initiator, and a heteroaromatic diketo
This type of initiating system contains a metallocene, an initiator, and a heteroaromatic diketo carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
. Metallocenes in combination with initiators accelerate polymerization of poly(methyl methacrylate) and produce a polymer with a narrower molecular weight distribution. The example shown here consists of indenylzirconium (a metallocene) and benzoyl peroxide (an initiator). Also, initiating systems containing heteroaromatic diketo carboxylic acids, such as 3,6-bis(''o''-carboxybenzoyl)-''N''-isopropylcarbazole in this example, are known to catalyze the decomposition of benzoyl peroxide. Initiating systems with this particular heteroaromatic diket carboxylic acid are also known to have effects on the microstructure
Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material (such as metals, polymers ...
of the polymer. The combination of all of these components—a metallocene, an initiator, and a heteroaromatic diketo carboxylic acid—yields a ternary initiating system that was shown to accelerate the polymerization and produce polymers with enhanced heat resistance and regular microstructure.
Initiator efficiency
Due to side reactions, not all of radicals from the dissociation of initiator molecules actually add monomers to form polymer chains. The efficiency factor ''f'' is defined as the fraction of the original initiator which contributes to the polymerization reaction. The maximal value of ''f'' is 1, but typical values range from 0.3 to 0.8. The following types of reactions can decrease the efficiency of the initiator. ;Primary recombination: Two radicals recombine before initiating a chain (Figure 8). This occurs within the solvent cage, meaning that no solvent has yet come between the new radicals. ;Other recombination pathways: Two radical initiators recombine before initiating a chain, but not in the solvent cage (Figure 9).
;Other recombination pathways: Two radical initiators recombine before initiating a chain, but not in the solvent cage (Figure 9).
 ;Side reactions: One radical is produced instead of the three radicals that could be produced (Figure 10).
;Side reactions: One radical is produced instead of the three radicals that could be produced (Figure 10).
Propagation
During polymerization, a polymer spends most of its time in increasing its chain length, or propagating. After theradical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical i ...
is formed, it attacks a monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
(Figure 11). In an ethene monomer, one electron pair is held securely between the two carbons in a sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools o ...
. The other is more loosely held in a pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbita ...
. The free radical uses one electron from the pi bond to form a more stable bond with the carbon atom. The other electron returns to the second carbon atom, turning the whole molecule into another radical. This begins the polymer chain. Figure 12 shows how the orbitals of an ethylene monomer interact with a radical initiator.

 Once a chain has been initiated, the chain propagates (Figure 13) until there are no more monomers (
Once a chain has been initiated, the chain propagates (Figure 13) until there are no more monomers (living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer ...
) or until termination occurs. There may be anywhere from a few to thousands of propagation steps depending on several factors such as radical and chain reactivity, the solvent, and temperature. The mechanism of chain propagation is as follows:

Termination
Chain termination
Chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.
Mechanisms of termination
In polymer chemist ...
is inevitable in radical polymerization due to the high reactivity of radicals. Termination can occur by several different mechanisms. If longer chains are desired, the initiator concentration should be kept low; otherwise, many shorter chains will result.
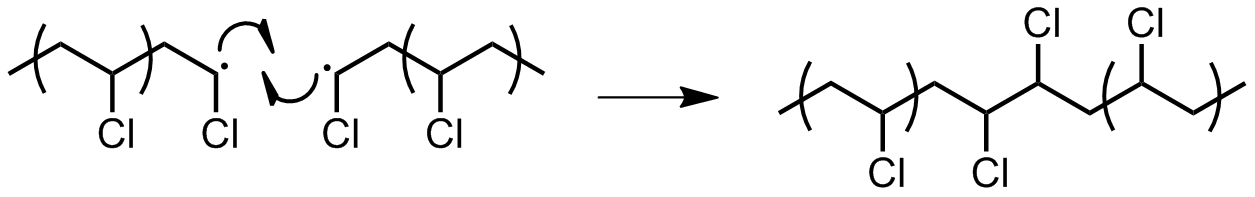
* Combination of two active chain ends: one or both of the following processes may occur.
** ''Combination:'' two chain ends simply couple together to form one long chain (Figure 14). One can determine if this mode of termination is occurring by monitoring the molecular weight of the propagating species: combination will result in doubling of molecular weight. Also, combination will result in a polymer that is C2 symmetric about the point of the combination.  ** '' Radical disproportionation:'' a hydrogen atom from one chain end is abstracted to another, producing a polymer with a terminal unsaturated group and a polymer with a terminal saturated group (Figure 15).
** '' Radical disproportionation:'' a hydrogen atom from one chain end is abstracted to another, producing a polymer with a terminal unsaturated group and a polymer with a terminal saturated group (Figure 15).  * Combination of an active chain end with an initiator radical (Figure 16).
* Combination of an active chain end with an initiator radical (Figure 16).  * Interaction with impurities or inhibitors.
* Interaction with impurities or inhibitors. Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
is the common inhibitor. The growing chain will react with molecular oxygen, producing an oxygen radical, which is much less reactive (Figure 17). This significantly slows down the rate of propagation. 
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor ...
, butylated hydroxyl toluene, and diphenyl picryl hydrazyl (DPPH
DPPH is a common abbreviation for the organic chemical compound 2,2-diphenyl-1-picrylhydrazyl. It is a dark-colored crystalline powder composed of stable free radical molecules. DPPH has two major applications, both in laboratory research: one is ...
, Figure 18) are a few other inhibitors. The latter is an especially effective inhibitor because of the resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
stabilization of the radical. 
Chain transfer
Contrary to the other modes of termination,chain transfer
Chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule.
:P• + XR' → PX + R'•
Chain transfer reactions reduce the average molecular weight of the final polymer. Chain ...
results in the destruction of only one radical, but also the creation of another radical. Often, however, this newly created radical is not capable of further propagation. Similar to disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term ca ...
, all chain-transfer mechanisms also involve the abstraction of a hydrogen or other atom. There are several types of chain-transfer mechanisms.
* ''To solvent:'' a hydrogen atom is abstracted from a solvent molecule, resulting in the formation of radical on the solvent molecules, which will not propagate further (Figure 19).  The effectiveness of chain transfer involving solvent molecules depends on the amount of solvent present (more solvent leads to greater probability of transfer), the strength of the bond involved in the abstraction step (weaker bond leads to greater probability of transfer), and the stability of the solvent radical that is formed (greater stability leads to greater probability of transfer). Halogens, except fluorine, are easily transferred.
* ''To monomer:'' a hydrogen atom is abstracted from a monomer. While this does create a radical on the affected monomer, resonance stabilization of this radical discourages further propagation (Figure 20).
The effectiveness of chain transfer involving solvent molecules depends on the amount of solvent present (more solvent leads to greater probability of transfer), the strength of the bond involved in the abstraction step (weaker bond leads to greater probability of transfer), and the stability of the solvent radical that is formed (greater stability leads to greater probability of transfer). Halogens, except fluorine, are easily transferred.
* ''To monomer:'' a hydrogen atom is abstracted from a monomer. While this does create a radical on the affected monomer, resonance stabilization of this radical discourages further propagation (Figure 20).  * ''To initiator:'' a polymer chain reacts with an initiator, which terminates that polymer chain, but creates a new radical initiator (Figure 21). This initiator can then begin new polymer chains. Therefore, contrary to the other forms of chain transfer, chain transfer to the initiator does allow for further propagation. Peroxide initiators are especially sensitive to chain transfer.
* ''To initiator:'' a polymer chain reacts with an initiator, which terminates that polymer chain, but creates a new radical initiator (Figure 21). This initiator can then begin new polymer chains. Therefore, contrary to the other forms of chain transfer, chain transfer to the initiator does allow for further propagation. Peroxide initiators are especially sensitive to chain transfer.  * ''To polymer:'' the radical of a polymer chain abstracts a hydrogen atom from somewhere on another polymer chain (Figure 22). This terminates the growth of one polymer chain, but allows the other to branch and resume growing. This reaction step changes neither the number of polymer chains nor the number of monomers which have been polymerized, so that the number-average
* ''To polymer:'' the radical of a polymer chain abstracts a hydrogen atom from somewhere on another polymer chain (Figure 22). This terminates the growth of one polymer chain, but allows the other to branch and resume growing. This reaction step changes neither the number of polymer chains nor the number of monomers which have been polymerized, so that the number-average degree of polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule.
For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by D ...
is unaffected. ''Effects of chain transfer:'' The most obvious effect of chain transfer is a decrease in the polymer chain length. If the rate of transfer is much larger than the rate of propagation, then very small polymers are formed with chain lengths of 2-5 repeating units (
''Effects of chain transfer:'' The most obvious effect of chain transfer is a decrease in the polymer chain length. If the rate of transfer is much larger than the rate of propagation, then very small polymers are formed with chain lengths of 2-5 repeating units (telomerization
Telomerization is a reaction that produces a particular kind of oligomer with two distinct end groups. The oligomer is called a telomer. Some telomerizations proceed by radical pathways, many do not. A generic equation is:
: A-B + n M -> A-M_-B ...
). The Mayo equation estimates the influence of chain transfer on chain length (''xn''): . Where ''ktr'' is the rate constant for chain transfer and ''kp'' is the rate constant for propagation. The Mayo equation assumes that transfer to solvent is the major termination pathway.
Methods
There are four industrial methods of radical polymerization: * ''Bulk polymerization
Bulk polymerization or mass polymerization is carried out by adding a soluble radical initiator to pure monomer in liquid state. The initiator should dissolve in the monomer. The reaction is initiated by heating or exposing to radiation. As the rea ...
:'' reaction mixture contains only initiator and monomer, no solvent.
* ''Solution polymerization
Solution polymerization is a method of industrial polymerization. In this procedure, a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator.
The reaction results in a polymer which is also soluble in the chosen sol ...
:'' reaction mixture contains solvent, initiator, and monomer.
* ''Suspension polymerization
Suspension polymerization is a heterogeneous radical polymerization process that uses mechanical agitation to mix a monomer or mixture of monomers in a liquid phase, such as water, while the monomers polymerize, forming spheres of polymer. The m ...
:'' reaction mixture contains an aqueous phase, water-insoluble monomer, and initiator soluble in the monomer droplets (both the monomer and the initiator are hydrophobic).
* ''Emulsion polymerization Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer ...
:'' similar to suspension polymerization except that the initiator is soluble in the aqueous phase rather than in the monomer droplets (the monomer is hydrophobic, and the initiator is hydrophilic). An emulsifying agent is also needed.
Other methods of radical polymerization include the following:
* ''Template polymerization'': In this process, polymer chains are allowed to grow along template macromolecules for the greater part of their lifetime. A well-chosen template can affect the rate of polymerization as well as the molar mass and microstructure of the daughter polymer. The molar mass of a daughter polymer can be up to 70 times greater than those of polymers produced in the absence of the template and can be higher in molar mass than the templates themselves. This is because of retardation of the termination for template-associated radicals and by hopping of a radical to the neighboring template after reaching the end of a template polymer.
* ''Plasma polymerization
Plasma polymerization (or glow discharge polymerization) uses plasma sources to generate a gas discharge that provides energy to activate or fragment gaseous or liquid monomer, often containing a vinyl group, in order to initiate polymerization. ...
'': The polymerization is initiated with plasma. A variety of organic molecules including alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s, alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
s, and alkane
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms tha ...
s undergo polymerization to high molecular weight products under these conditions. The propagation mechanisms appear to involve both ionic and radical species. Plasma polymerization offers a potentially unique method of forming thin polymer films for uses such as thin-film capacitors, antireflection coating
An antireflective, antiglare or anti-reflection (AR) coating is a type of optical coating applied to the surface of lenses, other optical elements, and photovoltaic cells to reduce reflection. In typical imaging systems, this improves the effic ...
s, and various types of thin membranes.
* ''Sonication'': The polymerization is initiated by high-intensity ultrasound. Polymerization to high molecular weight polymer is observed but the conversions are low (<15%). The polymerization is self-limiting because of the high viscosity produced even at low conversion. High viscosity hinders cavitation and radical production.
Reversible deactivation radical polymerization
Also known as living radical polymerization, controlled radical polymerization, reversible deactivation radical polymerization (RDRP) relies on completely pure reactions, preventing termination caused by impurities. Because these polymerizations stop only when there is no more monomer, polymerization can continue upon the addition of more monomer. Block copolymers can be made this way. RDRP allows for control of molecular weight and dispersity. However, this is very difficult to achieve and instead a pseudo-living polymerization occurs with only partial control of molecular weight and dispersity. ATRP and RAFT are the main types of complete radical polymerization. * ''Atom transfer radical polymerization Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal ...
(ATRP):'' based on the formation of a carbon-carbon bond by atom transfer radical addition. This method, independently discovered in 1995 by Mitsuo Sawamoto
Mitsuo Sawamoto (澤本 光男 ''Sawamoto Mitsuo'', December 12, 1954) is a Japanese chemist specializing in the field of polymer chemistry, Emeritus Professor at Kyoto University, professor at Chubu University.
He is co-recipient of the Frankli ...
and by Jin-Shan Wang and Krzysztof Matyjaszewski
Krzysztof "Kris" Matyjaszewski (; born April 8, 1950) is a Polish-American chemist. He is the J.C. Warner Professor of the Natural Sciences at the Carnegie Mellon University Matyjaszewski is best known for the discovery of atom transfer radical ...
, requires reversible activation of a dormant species (such as an alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
) and a transition metal halide catalyst (to activate dormant species).
* '' Reversible Addition-Fragmentation Chain-Transfer Polymerization (RAFT):'' requires a compound that can act as a reversible chain-transfer agent, such as dithio compound.
* ''Stable Free Radical Polymerization (SFRP)'': used to synthesize linear or branched polymers with narrow molecular weight distributions and reactive end groups on each polymer chain. The process has also been used to create block co-polymers with unique properties. Conversion rates are about 100% using this process but require temperatures of about 135 °C. This process is most commonly used with acrylates, styrenes, and dienes. The reaction scheme in Figure 23 illustrates the SFRP process. 
TEMPO
In musical terminology, tempo ( Italian, 'time'; plural ''tempos'', or ''tempi'' from the Italian plural) is the speed or pace of a given piece. In classical music, tempo is typically indicated with an instruction at the start of a piece (ofte ...
molecule (Figure 24), premature termination by coupling is reduced. As with all living polymerizations, the polymer chain grows until all of the monomer is consumed.
Kinetics
In typical chain growth polymerizations, the reaction rates for initiation, propagation and termination can be described as follows: :