Formylation Reactions on:
[Wikipedia]
[Google]
[Amazon]
 Formylation refers to any chemical processes in which a compound is functionalized with a formyl group (-CH=O). In organic chemistry, the term is most commonly used with regards to
Formylation refers to any chemical processes in which a compound is functionalized with a formyl group (-CH=O). In organic chemistry, the term is most commonly used with regards to
 Formylation of
Formylation of
 In bacteria and organelles, the initiation of protein synthesis is signaled by the formation of formyl-methionyl-tRNA (tRNAfMet). This reaction is dependent on 10-formyltetrahydrofolate, and the enzyme methionyl-tRNA formyltransferase.
This reaction is not used by eukaryotes or Archaea, as the presence of tRNAfMet in non bacterial cells is dubbed as intrusive material and quickly eliminated. After its production, tRNAfMet is delivered to the 30S subunit of the ribosome in order to start protein synthesis. fMet possesses the same codon sequence as methionine. However, fMet is only used for the initiation of protein synthesis and is thus found only at the N terminus of the protein. Methionine is used during the rest translation. In ''E. coli'', tRNAfMet is specifically recognized by initiation factor IF-2, as the formyl group blocks peptide bond formation at the N-terminus of methionine.
Once protein synthesis is accomplished, the formyl group on methionine can be removed by
In bacteria and organelles, the initiation of protein synthesis is signaled by the formation of formyl-methionyl-tRNA (tRNAfMet). This reaction is dependent on 10-formyltetrahydrofolate, and the enzyme methionyl-tRNA formyltransferase.
This reaction is not used by eukaryotes or Archaea, as the presence of tRNAfMet in non bacterial cells is dubbed as intrusive material and quickly eliminated. After its production, tRNAfMet is delivered to the 30S subunit of the ribosome in order to start protein synthesis. fMet possesses the same codon sequence as methionine. However, fMet is only used for the initiation of protein synthesis and is thus found only at the N terminus of the protein. Methionine is used during the rest translation. In ''E. coli'', tRNAfMet is specifically recognized by initiation factor IF-2, as the formyl group blocks peptide bond formation at the N-terminus of methionine.
Once protein synthesis is accomplished, the formyl group on methionine can be removed by 
 The formylation reaction is proposed to occur through a direct transfer reaction in which the amine group of GAR nucleophilically attacks N10-formyl-THF creating a tetrahedral intermediate. As the α-amino group of GAR is relatively reactive, deprotonation of the nucleophile is proposed to occur by solvent. In the active site, Asn 106, His 108, and Asp 144 are positioned to assist with formyl transfer. However, mutagenesis studies have indicated that these residues are not individually essential for catalysis, as only mutations of two or more residues inhibit the enzyme. Based on the structure the negatively charged Asp144 is believed to increase the pKa of His108, allowing the protonated imidazolium group of His108 to enhances the electrophillicity of the N10-formyl-THF formyl group. Additionally, His108 and Asn106 are believed to stabilize the oxyanion formed in the transition state.
The formylation reaction is proposed to occur through a direct transfer reaction in which the amine group of GAR nucleophilically attacks N10-formyl-THF creating a tetrahedral intermediate. As the α-amino group of GAR is relatively reactive, deprotonation of the nucleophile is proposed to occur by solvent. In the active site, Asn 106, His 108, and Asp 144 are positioned to assist with formyl transfer. However, mutagenesis studies have indicated that these residues are not individually essential for catalysis, as only mutations of two or more residues inhibit the enzyme. Based on the structure the negatively charged Asp144 is believed to increase the pKa of His108, allowing the protonated imidazolium group of His108 to enhances the electrophillicity of the N10-formyl-THF formyl group. Additionally, His108 and Asn106 are believed to stabilize the oxyanion formed in the transition state.


 The amine on AICAR is much less nucleophillic than its counterpart on GAR due to delocalization of electrons in AICAR through conjugation. Therefore, the N5 nucleophile of AIRCAR must be activated for the formylation reaction to occur. Histidine 268 and Lysine 267 have been found to be essential for catalysis and are conserved in all AICAR transformylase. Histidine 268 is involved in deprotonation of the N5 nucleophile of AICAR, whereas Lysine 267 is proposed to stabilize the tetrahedral intermediate.
The amine on AICAR is much less nucleophillic than its counterpart on GAR due to delocalization of electrons in AICAR through conjugation. Therefore, the N5 nucleophile of AIRCAR must be activated for the formylation reaction to occur. Histidine 268 and Lysine 267 have been found to be essential for catalysis and are conserved in all AICAR transformylase. Histidine 268 is involved in deprotonation of the N5 nucleophile of AICAR, whereas Lysine 267 is proposed to stabilize the tetrahedral intermediate.

 ε-Formylation is one of many
ε-Formylation is one of many 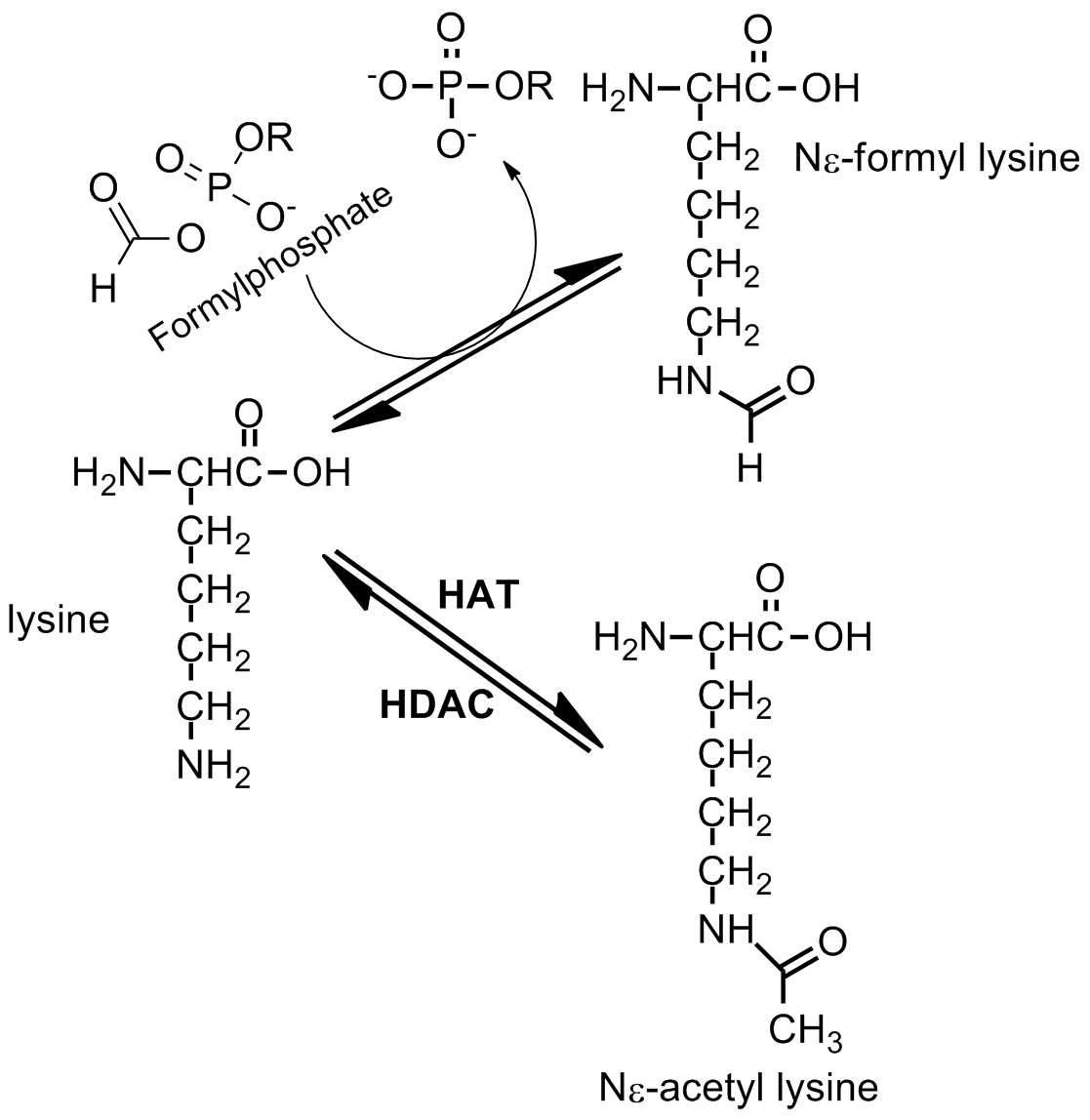 Formylation has been identified on the Nε of lysine residues in histones and proteins. This modification has been observed in linker histones and high mobility group proteins, it is highly abundant and it is believed to have a role in the epigenetics of chromatin function. Lysines that are formylated have been shown to play a role in DNA binding. Additionally, formylation has been detected on histone lysines that are also known to be acetylated and methylated. Thus, formylation may block other post-translational modifications.
Formylation is detected most frequently on 19 different modification sites on Histone H1. The genetic expression of the cell is highly disrupted by formylation, which may cause diseases such as cancer. The development of these modifications may be due to oxidative stress.
In histone proteins, lysine is typically modified by Histone Acetyl-Transferases (HATs) and Histone Deacetylases (HDAC or KDAC).
The acetylation of lysine is fundamental to the regulation and expression of certain genes. Oxidative stress creates a significantly different environment in which acetyl-lysine can be quickly outcompeted by the formation of formyl-lysine due to the high reactivity of formylphosphate species. This situation is currently believed to be caused by oxidative DNA damage.
A mechanism for the formation of formylphosphate has been proposed, which it is highly dependent on oxidatively damaged DNA and mainly driven by radical chemistry within the cell. The formylphosphate produced can then be used to formylate lysine. Oxidative stress is believed to play a role in the availability of lysine residues in the surface of proteins and the possibility of being formylated.
Formylation has been identified on the Nε of lysine residues in histones and proteins. This modification has been observed in linker histones and high mobility group proteins, it is highly abundant and it is believed to have a role in the epigenetics of chromatin function. Lysines that are formylated have been shown to play a role in DNA binding. Additionally, formylation has been detected on histone lysines that are also known to be acetylated and methylated. Thus, formylation may block other post-translational modifications.
Formylation is detected most frequently on 19 different modification sites on Histone H1. The genetic expression of the cell is highly disrupted by formylation, which may cause diseases such as cancer. The development of these modifications may be due to oxidative stress.
In histone proteins, lysine is typically modified by Histone Acetyl-Transferases (HATs) and Histone Deacetylases (HDAC or KDAC).
The acetylation of lysine is fundamental to the regulation and expression of certain genes. Oxidative stress creates a significantly different environment in which acetyl-lysine can be quickly outcompeted by the formation of formyl-lysine due to the high reactivity of formylphosphate species. This situation is currently believed to be caused by oxidative DNA damage.
A mechanism for the formation of formylphosphate has been proposed, which it is highly dependent on oxidatively damaged DNA and mainly driven by radical chemistry within the cell. The formylphosphate produced can then be used to formylate lysine. Oxidative stress is believed to play a role in the availability of lysine residues in the surface of proteins and the possibility of being formylated.

 Inhibition of enzymes involved in purine biosynthesis has been exploited as a potential drug target for chemotherapy.
Cancer cells require high concentrations of purines to facilitate division and tend to rely on de novo synthesis rather than the nucleotide salvage pathway. Several folate based inhibitors have been developed to inhibit formylation reactions by GAR transformylase and AICAR transformylase. The first GAR transformylase inhibitor Lometrexol 6R)5,10-dideazatetrahydrofolatewas developed in the 1980s through a collaboration between
Inhibition of enzymes involved in purine biosynthesis has been exploited as a potential drug target for chemotherapy.
Cancer cells require high concentrations of purines to facilitate division and tend to rely on de novo synthesis rather than the nucleotide salvage pathway. Several folate based inhibitors have been developed to inhibit formylation reactions by GAR transformylase and AICAR transformylase. The first GAR transformylase inhibitor Lometrexol 6R)5,10-dideazatetrahydrofolatewas developed in the 1980s through a collaboration between
aromatic compound
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
s (for example the conversion of benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
to benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
in the Gattermann–Koch reaction
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) ...
). In biochemistry the reaction is catalysed by enzymes such as formyltransferases.
Formylation generally involves the use of formylation agents, reagents that give rise to the CHO group. Among the many formylation reagents, particularly important are formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
and carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
. A formylation reaction in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
refers to organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
s in which an organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
is functionalized with a formyl group (-CH=O). The reaction is a route to aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s (''C''-CH=O), formamide
Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, ...
s (''N''-CH=O), and formate esters
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.
Fundamentals
When dissolved in water, formic acid co ...
(''O''-CH=O).
Formylation agents
A reagent that delivers the formyl group is called a formylating agent. *Formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
* Dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liqui ...
and phosphorus oxychloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phos ...
in the Vilsmeier-Haack reaction.
* Hexamethylenetetramine
Hexamethylenetetramine (HMTA), also known as 1,3,5,7-tetraazaadamantane, is a heterocyclic organic compound with diverse applications. It has the chemical formula (CH2)6N4 and is a white crystalline compound that is highly soluble in water and p ...
in the Duff reaction
The Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source. The method is generally inefficient. The reaction is named after J ...
and the Sommelet reaction
The Sommelet reaction is an organic reaction in which a benzyl halide is converted to an aldehyde by action of hexamine and water. It is named after the French chemist Marcel Sommelet, who first reported the reaction in 1913.
:
One example, thi ...
* Carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
and hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
in the Gattermann-Koch reaction
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) ...
* Cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
s in the Gattermann reaction
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) ...
. This method synthesizes aromatic aldehydes using hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
and hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
(or another metallic cyanide as such zinc cyanide
Zinc cyanide is the inorganic compound with the formula Zn( CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.
Structure
In Zn(CN)2, zinc adop ...
) in the presence of Lewis acid catalysts:
* Chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
in the Reimer-Tiemann reaction
* Dichloromethyl methyl ether in Rieche formylation
A particularly important formylation process is hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
, which converts alkenes to the homologated aldehyde.
Aromatic formylation
Formylation reactions are a form ofelectrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
and therefore work best with electron-rich starting materials. Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
s are a common substrate, as they readily deprotonate to excellent phenoxide
Phenolates (also called phenoxides) are anions, salts, and esters of phenols, containing the phenolate ion. They may be formed by reaction of phenols with strong base.
Properties
Alkali metal phenolates, such as sodium phenolate hydrolyze in aq ...
nucleophiles. Other electron-rich substrates, such as mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenze ...
, pyrrole
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrol ...
, or fused aromatic rings can also be expected to react. Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
will react under aggressive conditions but deactivated rings such as pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
are difficult to formylate effectively.
Many formylation reactions will select only the ''ortho'' product (e.g. salicylaldehyde
Salicylic aldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula . Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almo ...
), attributed to attraction between the phenoxide and the formylating reagent. Ionic interactions have been invoked for the cationic nitrogen centres in the Vilsmeier–Haack reaction and Duff reaction
The Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source. The method is generally inefficient. The reaction is named after J ...
, and the electron-deficient carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
in the Reimer-Tiemann reaction; coordination to high oxidation metals has been invoked in the Casiraghi and Rieche formylations (cf. Kolbe–Schmitt reaction
The Kolbe–Schmitt reaction or Kolbe process (named after Hermann Kolbe and Rudolf Schmitt) is a carboxylation chemical reaction that proceeds by treating phenol with sodium hydroxide to form sodium phenoxide, then heating sodium phenoxide wi ...
).
The direct reaction between phenol and paraformaldehyde
Paraformaldehyde (PFA) is the smallest polyoxymethylene, the polymerization product of formaldehyde with a typical degree of polymerization of 8–100 units. Paraformaldehyde commonly has a slight odor of formaldehyde due to decomposition. Par ...
is possible via the Casiraghi formylation, but other methods apply masked forms of formaldehyde, in part to limit the formation of phenol formaldehyde resin
Phenol formaldehyde resins (PF), also called phenolic resins or phenoplasts, are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commercial syntheti ...
s. Aldehydes are strongly deactivating and as such phenols typically only react once. However certain reactions, such as the Duff reaction
The Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source. The method is generally inefficient. The reaction is named after J ...
, can give double addition.
Formylation can be applied to other aromatic rings. As it generally begins with nucleophilic attack by the aromatic group, the electron density of the ring is an important factor. Some aromatic compounds, such as pyrrole, are known to formylate regioselectively.
Formylation of benzene rings can be achieved via the Gattermann reaction
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) ...
and Gattermann-Koch reaction
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) ...
. These involve strong acid catalysis and proceed in a manner similar to the Friedel–Crafts reaction
The Friedel–Crafts reactions are a set of organic reaction, reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an Aromatic hydrocarbon, aromatic ring. Friedel–Crafts reactions are of two main types: alky ...
.
Aliphatic formylation
Hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
of alkenes is the most important method for obtaining aliphatic formyls (i.e., aldehydes). The reaction is largely restricted to industrial settings. Several specialty methods exist for laboratory-scale synthesis, including the Sommelet reaction
The Sommelet reaction is an organic reaction in which a benzyl halide is converted to an aldehyde by action of hexamine and water. It is named after the French chemist Marcel Sommelet, who first reported the reaction in 1913.
:
One example, thi ...
, Bouveault aldehyde synthesis or Bodroux–Chichibabin aldehyde synthesis The Bodroux–Chichibabin aldehyde synthesis is a chemical reaction whereby a Grignard reagent is converted to an aldehyde one carbon longer.
:
Reaction of a Grignard reagent with triethyl orthoformate gives an acetal, which can be hydrolyzed
...
.
Formylation reactions in biology
In biochemistry, the addition of a formyl functional group is termed "formylation". A formyl functional group consists of a carbonyl bonded to hydrogen. When attached to an R group, a formyl group is called analdehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
.
Formylation has been identified in several critical biological processes. Methionine was first discovered to be formylated in ''E. coli'' by Marcker and Sanger in 1964 and was later identified to be involved in the initiation of protein synthesis in bacteria and organelles. The formation of ''N''-formylmethionine is catalyzed by the enzyme methionyl-tRNA transformylase. Additionally, two formylation reactions occur in the de novo biosynthesis of purines. These reactions are catalyzed by the enzymes glycinamide ribonucleotide (GAR) transformylase and 5-aminoimidazole-4-carboxyamide ribotide (AICAR) transformylase. More recently, formylation has been discovered to be a histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei and in most Archaeal phyla. They act as spools around which DNA winds to create structural units called nucleosomes ...
modification, which may modulate gene expression.
Methanogenesis
 Formylation of
Formylation of methanofuran
Methanofurans (MFRs) are a family of chemical compounds found in methanogenic archaea. These species feature a 2-aminomethylfuran linked to phenoxy group. At least three different end groups are recognized: R = tricarboxyheptanoyl (methanofuran ...
initiates the methanogenesis
Methanogenesis or biomethanation is the formation of methane coupled to energy conservation by microbes known as methanogens. It is the fourth and final stage of anaerobic digestion. Organisms capable of producing methane for energy conservation h ...
cycle. The formyl group is derived from carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and is converted to methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
.
Formylation in protein synthesis
 In bacteria and organelles, the initiation of protein synthesis is signaled by the formation of formyl-methionyl-tRNA (tRNAfMet). This reaction is dependent on 10-formyltetrahydrofolate, and the enzyme methionyl-tRNA formyltransferase.
This reaction is not used by eukaryotes or Archaea, as the presence of tRNAfMet in non bacterial cells is dubbed as intrusive material and quickly eliminated. After its production, tRNAfMet is delivered to the 30S subunit of the ribosome in order to start protein synthesis. fMet possesses the same codon sequence as methionine. However, fMet is only used for the initiation of protein synthesis and is thus found only at the N terminus of the protein. Methionine is used during the rest translation. In ''E. coli'', tRNAfMet is specifically recognized by initiation factor IF-2, as the formyl group blocks peptide bond formation at the N-terminus of methionine.
Once protein synthesis is accomplished, the formyl group on methionine can be removed by
In bacteria and organelles, the initiation of protein synthesis is signaled by the formation of formyl-methionyl-tRNA (tRNAfMet). This reaction is dependent on 10-formyltetrahydrofolate, and the enzyme methionyl-tRNA formyltransferase.
This reaction is not used by eukaryotes or Archaea, as the presence of tRNAfMet in non bacterial cells is dubbed as intrusive material and quickly eliminated. After its production, tRNAfMet is delivered to the 30S subunit of the ribosome in order to start protein synthesis. fMet possesses the same codon sequence as methionine. However, fMet is only used for the initiation of protein synthesis and is thus found only at the N terminus of the protein. Methionine is used during the rest translation. In ''E. coli'', tRNAfMet is specifically recognized by initiation factor IF-2, as the formyl group blocks peptide bond formation at the N-terminus of methionine.
Once protein synthesis is accomplished, the formyl group on methionine can be removed by peptide deformylase
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty ...
. The methionine residue can be further removed by the enzyme methionine aminopeptidase.

Formylation reactions in purine biosynthesis
Two formylation reactions are required in the eleven step de novo synthesis of inosine monophosphate (IMP), the precursor of the purine ribonucleotides AMP and GMP. Glycinamide ribonucleotide (GAR) transformylase catalyzes the formylation of GAR to formylglycinamidine ribotide (FGAR) in the fourth reaction of the pathway. In the penultimate step of de novo purine biosynthesis, 5-aminoimidazole-4-carboxyamide ribotide (AICAR) is formylated to 5-formaminoimidazole-4-carboxamide ribotide (FAICAR) byAICAR transformylase
In enzymology, a phosphoribosylaminoimidazolecarboxamide formyltransferase (), also known by the shorter name AICAR transformylase, is an enzyme that catalyzes the chemical reaction
:10-formyltetrahydrofolate + AICAR \rightleftharpoons tetrahydro ...
.
GAR transformylase
PurN GAR transformylase is found in eukaryotes and prokaryotes. However, a second GAR transformylase, PurT GAR transformylase has been identified in ''E. coli''. While the two enzymes have no sequence conservation and require different formyl donors, the specific activity and Km for GAR are the same in both PurT and PurN GAR transformylase.=PurN GAR transformylase
= PurN GAR transformylase 1CDE uses the coenzyme N10-formyltetrahydrofolate (N10-formyl-THF) as a formyl donor to formylate the α-amino group of GAR. In eukaryotes, PurN GAR transformylase is part of a large multifunctional protein, but is found as a single protein in prokaryotes.=Mechanism
= The formylation reaction is proposed to occur through a direct transfer reaction in which the amine group of GAR nucleophilically attacks N10-formyl-THF creating a tetrahedral intermediate. As the α-amino group of GAR is relatively reactive, deprotonation of the nucleophile is proposed to occur by solvent. In the active site, Asn 106, His 108, and Asp 144 are positioned to assist with formyl transfer. However, mutagenesis studies have indicated that these residues are not individually essential for catalysis, as only mutations of two or more residues inhibit the enzyme. Based on the structure the negatively charged Asp144 is believed to increase the pKa of His108, allowing the protonated imidazolium group of His108 to enhances the electrophillicity of the N10-formyl-THF formyl group. Additionally, His108 and Asn106 are believed to stabilize the oxyanion formed in the transition state.
The formylation reaction is proposed to occur through a direct transfer reaction in which the amine group of GAR nucleophilically attacks N10-formyl-THF creating a tetrahedral intermediate. As the α-amino group of GAR is relatively reactive, deprotonation of the nucleophile is proposed to occur by solvent. In the active site, Asn 106, His 108, and Asp 144 are positioned to assist with formyl transfer. However, mutagenesis studies have indicated that these residues are not individually essential for catalysis, as only mutations of two or more residues inhibit the enzyme. Based on the structure the negatively charged Asp144 is believed to increase the pKa of His108, allowing the protonated imidazolium group of His108 to enhances the electrophillicity of the N10-formyl-THF formyl group. Additionally, His108 and Asn106 are believed to stabilize the oxyanion formed in the transition state.

=PurT GAR transformylase
= PurT GAR transformylase requires formate as the formyl donor and ATP for catalysis. It has been estimated that PurT GAR transformylase carries out 14-50% of GAR formylations in ''E. coli''. The enzyme is a member of the ATP-grasp superfamily of proteins.=Mechanism
= A sequential mechanism has been proposed for PurT GAR transformylase in which a short lived formyl phosphate intermediate is proposed to first form. This formyl phosphate intermediate then undergoes nucleophilic attack by the GAR amine for transfer of the formyl group. A formyl phosphate intermediate has been detected in mutagenesis experiments, in which the mutant PurT GAR transforymylase had a weak affinity for formate. Incubating PurT GAR transformylase with formyl phosphate, ADP, and GAR, yields both ATP and FGAR. This further indicating that formyl phosphate may be an intermediate, as it is kinetically and chemically competent to carry out the formylation reaction in the enzyme. An enzyme phosphate intermediate preceding the formylphosphate intermediate has also been proposed to form based on positional isotope exchange studies. However, structural data indicates that the formate may be positioned for a direct attack on the γ-phosphate of ATP in the enzyme's active site to form the formylphosphate intermediate.
AICAR transformylase
AICAR transformylase requires the coenzyme N10-formyltetrahydrofolate (N10-formyl-THF) as the formyl donor for the formylation of AICAR to FAICAR. However, AICAR transformylase and GAR transformylase do not share a high sequence similarity or structural homology.=Mechanism
= The amine on AICAR is much less nucleophillic than its counterpart on GAR due to delocalization of electrons in AICAR through conjugation. Therefore, the N5 nucleophile of AIRCAR must be activated for the formylation reaction to occur. Histidine 268 and Lysine 267 have been found to be essential for catalysis and are conserved in all AICAR transformylase. Histidine 268 is involved in deprotonation of the N5 nucleophile of AICAR, whereas Lysine 267 is proposed to stabilize the tetrahedral intermediate.
The amine on AICAR is much less nucleophillic than its counterpart on GAR due to delocalization of electrons in AICAR through conjugation. Therefore, the N5 nucleophile of AIRCAR must be activated for the formylation reaction to occur. Histidine 268 and Lysine 267 have been found to be essential for catalysis and are conserved in all AICAR transformylase. Histidine 268 is involved in deprotonation of the N5 nucleophile of AICAR, whereas Lysine 267 is proposed to stabilize the tetrahedral intermediate.

Formylation in histone proteins
 ε-Formylation is one of many
ε-Formylation is one of many post-translational modifications
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translation (biolog ...
that occur on histone proteins, which been shown to modulate chromatin
Chromatin is a complex of DNA and protein found in eukaryote, eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important r ...
conformations and gene activation.
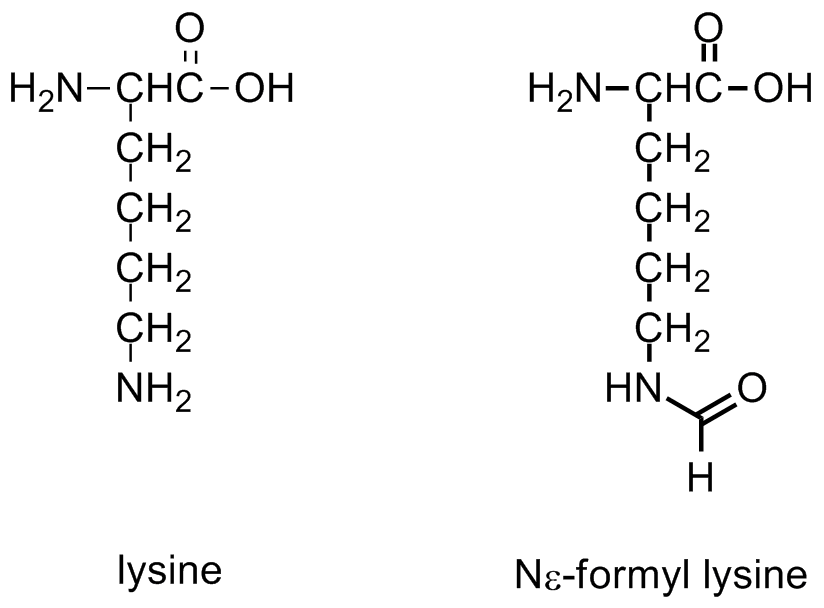
 Formylation has been identified on the Nε of lysine residues in histones and proteins. This modification has been observed in linker histones and high mobility group proteins, it is highly abundant and it is believed to have a role in the epigenetics of chromatin function. Lysines that are formylated have been shown to play a role in DNA binding. Additionally, formylation has been detected on histone lysines that are also known to be acetylated and methylated. Thus, formylation may block other post-translational modifications.
Formylation is detected most frequently on 19 different modification sites on Histone H1. The genetic expression of the cell is highly disrupted by formylation, which may cause diseases such as cancer. The development of these modifications may be due to oxidative stress.
In histone proteins, lysine is typically modified by Histone Acetyl-Transferases (HATs) and Histone Deacetylases (HDAC or KDAC).
The acetylation of lysine is fundamental to the regulation and expression of certain genes. Oxidative stress creates a significantly different environment in which acetyl-lysine can be quickly outcompeted by the formation of formyl-lysine due to the high reactivity of formylphosphate species. This situation is currently believed to be caused by oxidative DNA damage.
A mechanism for the formation of formylphosphate has been proposed, which it is highly dependent on oxidatively damaged DNA and mainly driven by radical chemistry within the cell. The formylphosphate produced can then be used to formylate lysine. Oxidative stress is believed to play a role in the availability of lysine residues in the surface of proteins and the possibility of being formylated.
Formylation has been identified on the Nε of lysine residues in histones and proteins. This modification has been observed in linker histones and high mobility group proteins, it is highly abundant and it is believed to have a role in the epigenetics of chromatin function. Lysines that are formylated have been shown to play a role in DNA binding. Additionally, formylation has been detected on histone lysines that are also known to be acetylated and methylated. Thus, formylation may block other post-translational modifications.
Formylation is detected most frequently on 19 different modification sites on Histone H1. The genetic expression of the cell is highly disrupted by formylation, which may cause diseases such as cancer. The development of these modifications may be due to oxidative stress.
In histone proteins, lysine is typically modified by Histone Acetyl-Transferases (HATs) and Histone Deacetylases (HDAC or KDAC).
The acetylation of lysine is fundamental to the regulation and expression of certain genes. Oxidative stress creates a significantly different environment in which acetyl-lysine can be quickly outcompeted by the formation of formyl-lysine due to the high reactivity of formylphosphate species. This situation is currently believed to be caused by oxidative DNA damage.
A mechanism for the formation of formylphosphate has been proposed, which it is highly dependent on oxidatively damaged DNA and mainly driven by radical chemistry within the cell. The formylphosphate produced can then be used to formylate lysine. Oxidative stress is believed to play a role in the availability of lysine residues in the surface of proteins and the possibility of being formylated.

Formylation in medicine
Formylation reactions as a drug target
 Inhibition of enzymes involved in purine biosynthesis has been exploited as a potential drug target for chemotherapy.
Cancer cells require high concentrations of purines to facilitate division and tend to rely on de novo synthesis rather than the nucleotide salvage pathway. Several folate based inhibitors have been developed to inhibit formylation reactions by GAR transformylase and AICAR transformylase. The first GAR transformylase inhibitor Lometrexol 6R)5,10-dideazatetrahydrofolatewas developed in the 1980s through a collaboration between
Inhibition of enzymes involved in purine biosynthesis has been exploited as a potential drug target for chemotherapy.
Cancer cells require high concentrations of purines to facilitate division and tend to rely on de novo synthesis rather than the nucleotide salvage pathway. Several folate based inhibitors have been developed to inhibit formylation reactions by GAR transformylase and AICAR transformylase. The first GAR transformylase inhibitor Lometrexol 6R)5,10-dideazatetrahydrofolatewas developed in the 1980s through a collaboration between Eli Lilly
Eli Lilly (July 8, 1838 – June 6, 1898) was a Union Army officer, pharmacist, chemist, and businessman who founded Eli Lilly and Company.
Lilly enlisted in the Union Army during the American Civil War and recruited a company of men to ...
and academic laboratories.
Although similar in structure to N10-formyl-THF, lometrexol is incapable of carrying out one carbon transfer reactions. Additionally, several GAR based inhibitors of GAR transformylase have also been synthesized.
Development of folate based inhibitors have been found to be particularly challenging as the inhibitors also down regulate the enzyme folypolyglutamate synthase, which adds additional γ-glutamates to monoglutamate folates and antifolates after entering the cell for increased enzyme affinity. This increased affinity can lead to antifolate resistance.
Leigh syndrome
Leigh syndrome
Leigh syndrome (also called Leigh disease and subacute necrotizing encephalomyelopathy) is an inherited neurometabolic disorder that affects the central nervous system. It is named after Archibald Denis Leigh, a British neuropsychiatrist who fir ...
is a neurodegenerative disorder that has been linked to a defect in an enzymatic formylation reaction. Leigh syndrome is typically associated with defects in oxidative phosphorylation, which occurs in the mitochondria. Exome sequencing
Exome sequencing, also known as whole exome sequencing (WES), is a genomic technique for sequencing all of the protein-coding regions of genes in a genome (known as the exome). It consists of two steps: the first step is to select only the subs ...
, has been used to identify a mutation in the gene coding for mitochondrial methionyl-tRNA formyltransferase (MTFMT) in patients with Leigh syndrome. The c.626C>T mutation identified in MTFMT yielding symptoms of Leigh Syndrome is believed to alter exon splicing leading to a frameshift mutation and a premature stop codon. Individuals with the MTFMT c.626C>T mutation were found to have reduced fMet-tRNAMet levels and changes in the formylation level of mitochondrically translated COX1. This link provides evidence for the necessity of formylated methionine in initiation of expression for certain mitochondrial genes.
See also
*Hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
* Hydroacylation
Hydroacylation is a type of organic reaction in which an electron-richSmith (2020), ''March's Organic Chemistry'', 8th ed. Rxn. 15-30. Unsaturated hydrocarbons, unsaturated hydrocarbon inserts into a formyl C-H bond. With alkenes, the p ...
References
See also
* ''N''-Formylmethionine {{Protein posttranslational modification Proteins Post-translational modification