formose reaction on:
[Wikipedia]
[Google]
[Amazon]
The formose reaction, discovered by

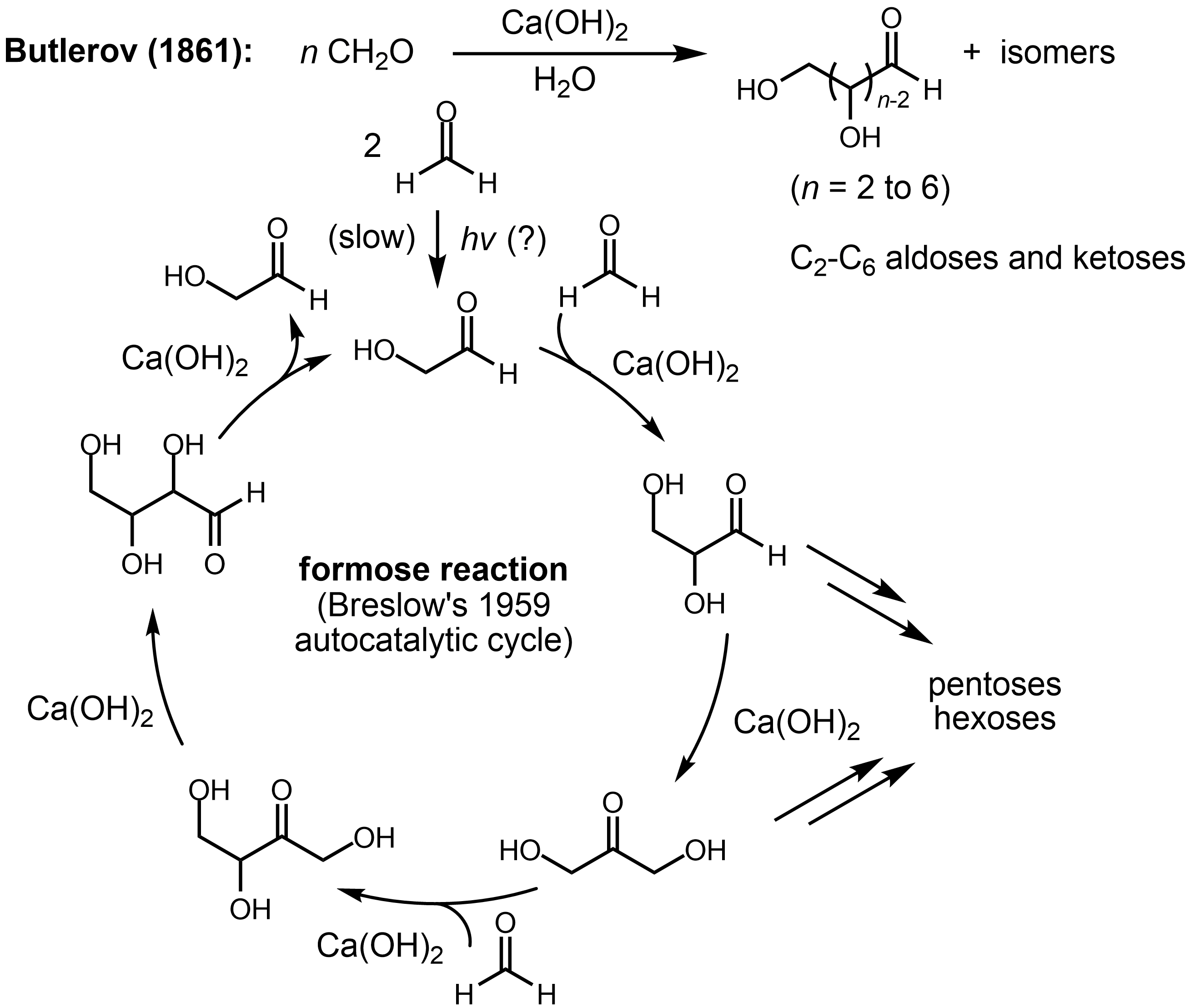 The reaction exhibits an induction period, during which only the nonproductive Cannizzaro disproportionation of formaldehyde (to methanol and formate) occurs. The initial dimerization of formaldehyde to give glycolaldehyde (1) occurs via an unknown mechanism, possibly promoted by light or through a free radical process and is very slow. However, the reaction is autocatalytic: 1 catalyzes the condensation of two molecules of formaldehyde to produce an additional molecule of 1. Hence, even a trace (as low as 3 ppm) of glycolaldehyde is enough to initiate the reaction. The autocatalytic cycle begins with the aldol reaction of 1 with formaldehyde to make glyceraldehyde (2). An aldose-ketose isomerization of 2 forms
The reaction exhibits an induction period, during which only the nonproductive Cannizzaro disproportionation of formaldehyde (to methanol and formate) occurs. The initial dimerization of formaldehyde to give glycolaldehyde (1) occurs via an unknown mechanism, possibly promoted by light or through a free radical process and is very slow. However, the reaction is autocatalytic: 1 catalyzes the condensation of two molecules of formaldehyde to produce an additional molecule of 1. Hence, even a trace (as low as 3 ppm) of glycolaldehyde is enough to initiate the reaction. The autocatalytic cycle begins with the aldol reaction of 1 with formaldehyde to make glyceraldehyde (2). An aldose-ketose isomerization of 2 forms
Aleksandr Butlerov
Alexander Mikhaylovich Butlerov (; 15 September 1828 – 17 August 1886) was a Russian chemist, one of the principal creators of the theory of chemical structure (1857–1861), the first to incorporate double bonds into structural for ...
in 1861, and hence also known as the Butlerov reaction, involves the formation of sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
s from formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
. The term formose is a portmanteau
In linguistics, a blend—also known as a blend word, lexical blend, or portmanteau—is a word formed by combining the meanings, and parts of the sounds, of two or more words together.
of formaldehyde and -ose (a suffix that means "sugar").
Reaction and mechanism
The reaction is catalyzed by a base and a divalent metal such ascalcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
. The intermediary steps taking place are aldol reaction
The aldol reaction (aldol addition) is a Chemical reaction, reaction in organic chemistry that combines two Carbonyl group, carbonyl compounds (e.g. aldehydes or ketones) to form a new β-hydroxy carbonyl compound. Its simplest form might invol ...
s, reverse aldol reactions, and aldose-ketose isomerizations. Intermediates are glycolaldehyde
Glycolaldehyde is the organic compound with the formula . It is the smallest possible molecule that contains both an aldehyde group () and a hydroxyl, hydroxyl group (). It is a highly Reactivity (chemistry), reactive molecule that occurs both ...
, glyceraldehyde
Glyceraldehyde (glyceral) is a triose monosaccharide with chemical formula C3 H6 O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes ...
, dihydroxyacetone
Dihydroxyacetone (; DHA), also known as glycerone, is a simple saccharide (a triose) with formula .
DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, ...
, and tetrose
In organic chemistry, a tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde () functional group in position 1 (aldotetroses) or a ketone () group in position 2 (ketotetroses).
File:DErythrose Fischer.svg , D-Erythrose
Fi ...
sugars. In 1959, Breslow proposed a mechanism for the reaction, consisting of the following steps:

 The reaction exhibits an induction period, during which only the nonproductive Cannizzaro disproportionation of formaldehyde (to methanol and formate) occurs. The initial dimerization of formaldehyde to give glycolaldehyde (1) occurs via an unknown mechanism, possibly promoted by light or through a free radical process and is very slow. However, the reaction is autocatalytic: 1 catalyzes the condensation of two molecules of formaldehyde to produce an additional molecule of 1. Hence, even a trace (as low as 3 ppm) of glycolaldehyde is enough to initiate the reaction. The autocatalytic cycle begins with the aldol reaction of 1 with formaldehyde to make glyceraldehyde (2). An aldose-ketose isomerization of 2 forms
The reaction exhibits an induction period, during which only the nonproductive Cannizzaro disproportionation of formaldehyde (to methanol and formate) occurs. The initial dimerization of formaldehyde to give glycolaldehyde (1) occurs via an unknown mechanism, possibly promoted by light or through a free radical process and is very slow. However, the reaction is autocatalytic: 1 catalyzes the condensation of two molecules of formaldehyde to produce an additional molecule of 1. Hence, even a trace (as low as 3 ppm) of glycolaldehyde is enough to initiate the reaction. The autocatalytic cycle begins with the aldol reaction of 1 with formaldehyde to make glyceraldehyde (2). An aldose-ketose isomerization of 2 forms dihydroxyacetone
Dihydroxyacetone (; DHA), also known as glycerone, is a simple saccharide (a triose) with formula .
DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, ...
(3). A further aldol reaction of 3 with formaldehyde produces tetrulose (6), which undergoes another ketose-aldose isomerization to form aldotetrose 7 (either threose or erythrose). The retro-aldol reaction of 7 generates two molecules of 1, resulting in the net production of a molecule of 1 from two molecules of formaldehyde, catalyzed by 1 itself (autocatalysis
In chemistry, a chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same reaction. Many forms of autocatalysis are recognized.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and ...
). During this process, 3 can also react with 1 to form ribulose (4), which can isomerize to give rise to ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally occurring form, , is a component of the ribonucleotides from which RNA is built, and so this comp ...
(5), an important building block of ribonucleic acid
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins ( messenger RNA). RNA and deoxyr ...
. The reaction conditions must be carefully controlled, otherwise the alkaline conditions will cause the aldoses to undergo the Cannizzaro reaction
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.
...
.
The aldose-ketose isomerization steps are promoted by chelation to calcium. However, these steps have been shown to proceed through a hydride shift mechanism by isotope labeling studies, instead of via an intermediate enediolate, as previously proposed.
Significance
The formose reaction is of importance to the question of theorigin of life
Abiogenesis is the natural process by which life arises from abiotic component, non-living matter, such as simple organic compounds. The prevailing scientific hypothesis is that the transition from non-living to organism, living entities on ...
, as it leads from simple formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
to complex sugars like ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally occurring form, , is a component of the ribonucleotides from which RNA is built, and so this comp ...
, a building block of RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
. In one experiment simulating early Earth conditions, pentoses formed from mixtures of formaldehyde, glyceraldehyde, and borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
minerals such as colemanite (Ca2B6O115H2O) or kernite
Kernite, also known as rasorite, is a hydrated sodium borate hydroxide mineral with formula . It is a colorless to white mineral crystallizing in the monoclinic crystal system typically occurring as prismatic to acicular (crystal habit), acicular ...
(Na2B4O7). However, issues remain with both the thermodynamic and kinetic feasibility of binding pre-made sugars to a pre-made nucleobase, as well as a method to selectively employ ribose from the mixture. Both formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
and glycolaldehyde
Glycolaldehyde is the organic compound with the formula . It is the smallest possible molecule that contains both an aldehyde group () and a hydroxyl, hydroxyl group (). It is a highly Reactivity (chemistry), reactive molecule that occurs both ...
have been observed spectroscopically in outer space, making the formose reaction of particular interest to the field of astrobiology
Astrobiology (also xenology or exobiology) is a scientific field within the List of life sciences, life and environmental sciences that studies the abiogenesis, origins, Protocell, early evolution, distribution, and future of life in the univ ...
.
References
{{Reflist Biochemical reactions Carbohydrates Organic reactions Origin of life RNA Name reactions