fluoroform on:
[Wikipedia]
[Google]
[Amazon]
Fluoroform, or trifluoromethane, is the
 is a potent
is a potent
MSDS at Oxford University
MSDS at mathesontrigas.com
Coupling of fluoroform with aldehydes using an electrogenerated base
{{fluorine compounds Fluoroalkanes Halomethanes Refrigerants Fire suppression agents Greenhouse gases Trifluoromethyl compounds Hydrofluorocarbons
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula . It is a hydrofluorocarbon
Hydrofluorocarbons (HFCs) are synthetic organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air condit ...
as well as being a part of the haloforms, a class of compounds with the formula (X = halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
) with C3v symmetry
Symmetry () in everyday life refers to a sense of harmonious and beautiful proportion and balance. In mathematics, the term has a more precise definition and is usually used to refer to an object that is Invariant (mathematics), invariant und ...
. Fluoroform is used in diverse applications in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. It is not an ozone depleter but is a greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
.
Synthesis
About 20 million kg per year are produced industrially as both a by-product of and precursor to the manufacture ofTeflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from ...
. It is produced by reaction of chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
with HF:
:
It is also generated biologically in small amounts apparently by decarboxylation of trifluoroacetic acid
Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not ...
.
Historical
Fluoroform was first obtained by Maurice Meslans in the violent reaction ofiodoform
Iodoform (also known as triiodomethane) is the organoiodine compound with the chemical formula . It is a pale yellow, crystalline, volatile substance, with a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes refe ...
with dry silver fluoride Silver fluoride can refer to:
* Silver subfluoride (disilver monofluoride), Ag2F
* Silver(I) fluoride (silver monofluoride, argentous fluoride), AgF
* Silver(I,II) fluorides (disilver trifluoride, trisilver tetrafluoride) Ag2F3, Ag3F4
* Silver(I ...
in 1894. The reaction was improved by Otto Ruff by substitution of silver fluoride by a mixture of mercury fluoride and calcium fluoride. The exchange reaction works with iodoform and bromoform, and the exchange of the first two halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
atoms by fluorine is vigorous. By changing to a two step process, first forming a bromodifluoromethane in the reaction of antimony trifluoride with bromoform and finishing the reaction with mercury fluoride the first efficient synthesis method was found by Henne.
Industrial applications
is used in thesemiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
industry in plasma etching
Plasma etching is a form of plasma processing used to fabricate integrated circuits. It involves a high-speed stream of glow discharge (Plasma (physics), plasma) of an appropriate gas mixture being shot (in pulses) at a sample. The plasma source, ...
of silicon oxide and silicon nitride. Known as R-23 or HFC-23, it was also a useful refrigerant
A refrigerant is a working fluid used in the cooling, heating, or reverse cooling/heating cycles of air conditioning systems and heat pumps, where they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are ...
, sometimes as a replacement for chlorotrifluoromethane (CFC-13) and is a byproduct of its manufacture.
When used as a fire suppressant, the fluoroform carries the DuPont
Dupont, DuPont, Du Pont, duPont, or du Pont may refer to:
People
* Dupont (surname) Dupont, also spelled as DuPont, duPont, Du Pont, or du Pont is a French surname meaning "of the bridge", historically indicating that the holder of the surname re ...
trade name, FE-13. is recommended for this application because of its low toxicity, its low reactivity, and its high density. HFC-23 has been used in the past as a replacement for Halon 1301(CFC-13B1) in fire suppression systems as a total flooding gaseous fire suppression agent.
Organic chemistry
Fluoroform is weakly acidic with a pK''a'' = 25–28 and quite inert. Attempted deprotonation results in defluorination to generate and difluorocarbene (). Some organocopper and organocadmium compounds have been developed as trifluoromethylation reagents. Fluoroform is a precursor of the Ruppert-Prakash reagent , which is a source of the nucleophilic anion.Greenhouse gas
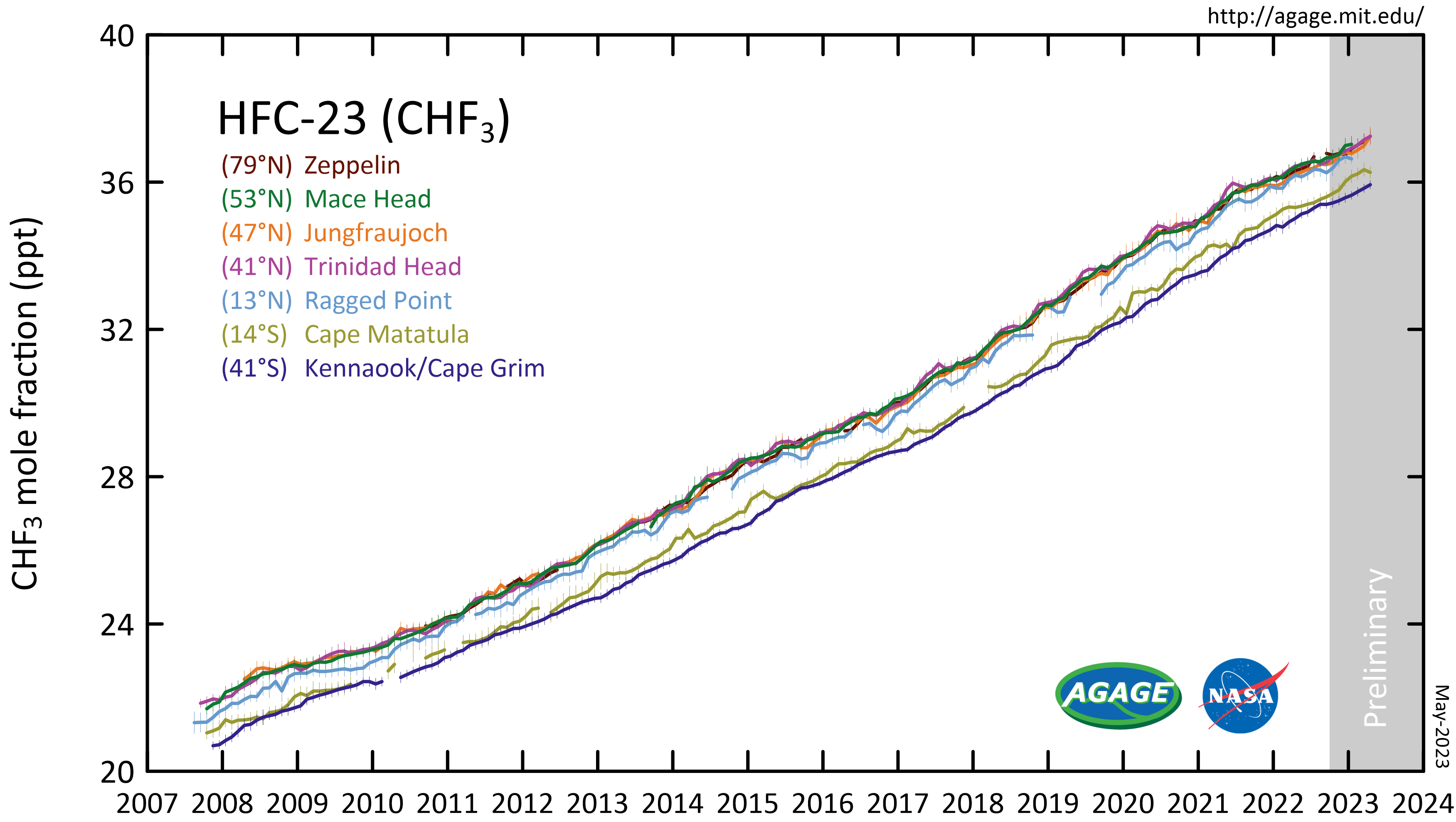
 is a potent
is a potent greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
. A ton of HFC-23 in the atmosphere has the same effect as 11,700 tons of carbon dioxide. This equivalency, also called a 100-yr global warming potential
Global warming potential (GWP) is a measure of how much heat a greenhouse gas traps in the atmosphere over a specific time period, relative to carbon dioxide (). It is expressed as a multiple of warming caused by the same mass of carbon dioxide ( ...
, is slightly larger at 14,800 for HFC-23.
The atmospheric lifetime is 270 years.
HFC-23 was the most abundant HFC in the global atmosphere until around 2001, when the global mean concentration of HFC-134a (1,1,1,2-tetrafluoroethane), the chemical now used extensively in automobile air conditioners, surpassed those of HFC-23. Global emissions of HFC-23 have in the past been dominated by the inadvertent production and release during the manufacture of the refrigerant HCFC-22 (chlorodifluoromethane).
Substantial decreases in HFC-23 emissions by developed countries were reported from the 1990s to the 2000s: from 6-8 Gg/yr in the 1990s to 2.8 Gg/yr in 2007.
However, research in 2024 strongly indicates that the HFC-23 emission decrease is much less than has been reported and does not meet the internationaly agreed Kigali Amendment
The Kigali Amendment to the Montreal Protocol is an international agreement to gradually reduce the consumption and production of hydrofluorocarbons (HFCs). It is a legally binding agreement designed to create rights and obligations in internati ...
of 2020.
The UNFCCC Clean Development Mechanism provided funding and facilitated the destruction of HFC-23.
Developing countries have become the largest producers of HCFC-23 in recent years according to data compiled by the Ozone Secretariat of the World Meteorological Organization. Emissions of all HFCs are included in the UNFCCCs Kyoto Protocol. To mitigate its impact, can be destroyed with electric plasma arc technologies or by high temperature incineration.
Additional physical properties
References
Literature
* * *External links
*MSDS at Oxford University
MSDS at mathesontrigas.com
Coupling of fluoroform with aldehydes using an electrogenerated base
{{fluorine compounds Fluoroalkanes Halomethanes Refrigerants Fire suppression agents Greenhouse gases Trifluoromethyl compounds Hydrofluorocarbons