Flame on:
[Wikipedia]
[Google]
[Amazon]
 A flame () is the visible, gaseous part of a
A flame () is the visible, gaseous part of a
 Color and temperature of a flame are dependent on the type of fuel involved in the combustion. For example, when a lighter is held to a
Color and temperature of a flame are dependent on the type of fuel involved in the combustion. For example, when a lighter is held to a
 Flame color depends on several factors, the most important typically being
Flame color depends on several factors, the most important typically being  In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about . The yellow arises from
In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about . The yellow arises from
 When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only an estimation of temperature. Other factors that determine its temperature are:
* In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion and the flame temperature is low, often just . This means that a lot of
When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only an estimation of temperature. Other factors that determine its temperature are:
* In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion and the flame temperature is low, often just . This means that a lot of
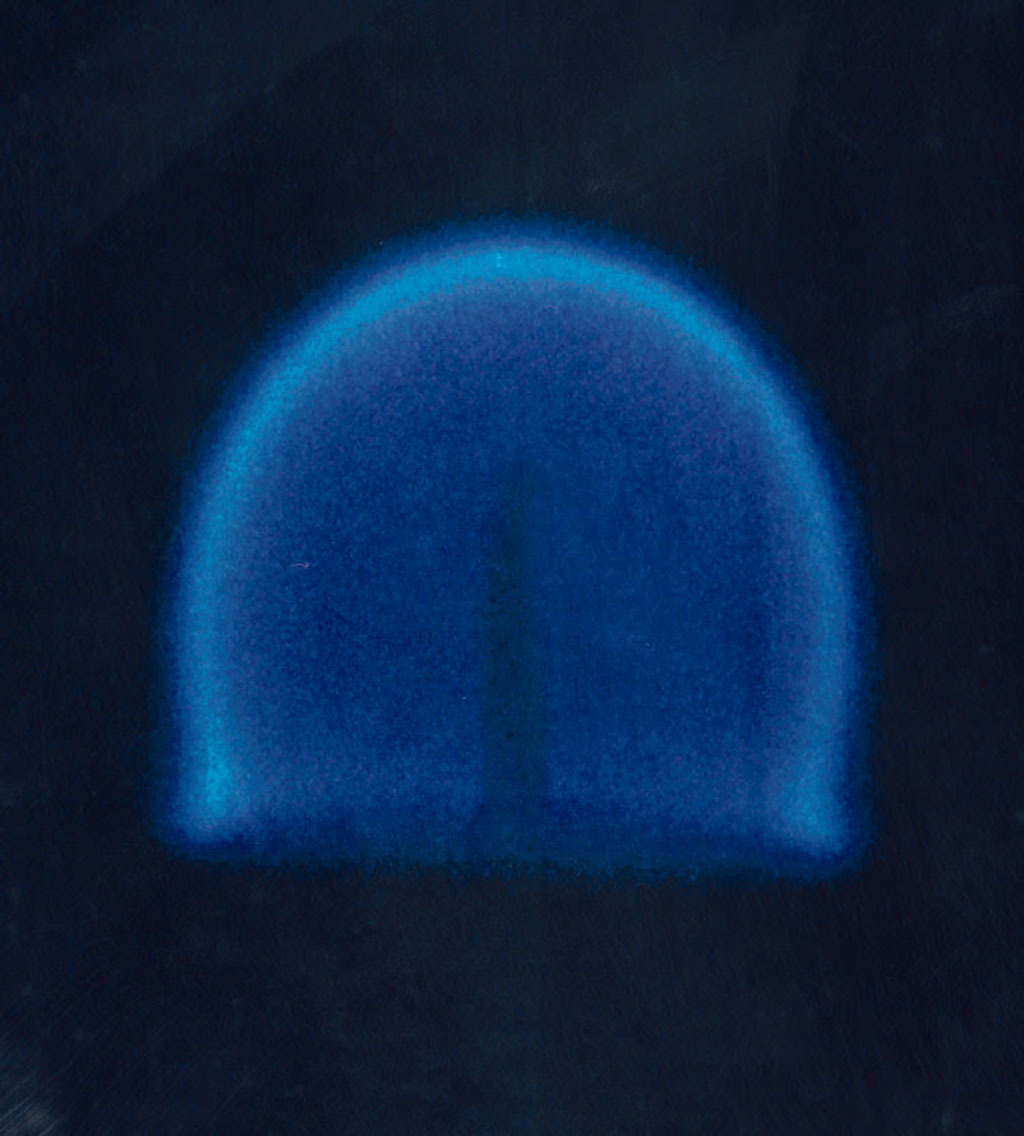 In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on
In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on
A candle flame strongly influenced and moved about by an electric field due to the flame having ions.
(archived 30 September 2011)
Ultra-Low Emissions Low-Swirl Burner
7 Shades of Fire
(archived 31 August 2017) * {{Authority control Fire lez:ЦӀай
 A flame () is the visible, gaseous part of a
A flame () is the visible, gaseous part of a fire
Fire is the rapid oxidation of a fuel in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products.
Flames, the most visible portion of the fire, are produced in the combustion re ...
. It is caused by a highly exothermic chemical reaction made in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density, they are then considered plasma.
Mechanism
candle
A candle is an ignitable candle wick, wick embedded in wax, or another flammable solid substance such as tallow, that provides light, and in some cases, a Aroma compound, fragrance. A candle can also provide heat or a method of keeping time. ...
, the applied heat causes the fuel molecules in the candle wax to vaporize. In this state they can then readily react with oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
in the air, which gives off enough heat in the subsequent exothermic reaction to vaporize yet more fuel, thus sustaining a consistent flame. The high temperature of the flame causes the vaporized fuel molecules to decompose, forming various incomplete combustion products and free radicals, and these products then react with each other and with the oxidizer involved in the reaction of the following flame (fire).
One may investigate different parts of a candle flame with the aid of a cold metal spoon: the higher parts of the flame produce water vapor deposition, the result of combustion, the yellow parts in the middle produce soot, and the area near the candle wick produces unburned wax. Goldsmiths use higher parts of a flame with a metallic blow-pipe for melting gold and silver. Sufficient energy in the flame will excite the electrons in some of the transient reaction intermediates such as the methylidyne radical (CH) and diatomic carbon (C2), which results in the emission of visible light as these substances release their excess energy (see spectrum below for an explanation of which specific radical species produce which specific colors). As the combustion temperature of a flame increases (if the flame contains small particles of unburnt carbon or other material), so does the average energy of the electromagnetic radiation given off by the flame (see Black body).
Other oxidizers besides oxygen can be used to produce a flame. Hydrogen burning in chlorine produces a flame and in the process emits gaseous hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
(HCl) as the combustion product. Another of many possible chemical combinations is hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
and nitrogen tetroxide which is hypergolic and commonly used in rocket engines. Fluoropolymers can be used to supply fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
as an oxidizer of metallic fuels, e.g. in the magnesium/teflon/viton composition.
The chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a ...
occurring in the flame are very complex and typically involve a large number of chemical reactions and intermediate species, most of them radicals. For instance, a well-known chemical kinetics scheme, GRI-Mech, uses 53 species and 325 elementary reactions to describe combustion of biogas
Biogas is a gaseous renewable energy source produced from raw materials such as agricultural waste, manure, municipal waste, plant material, sewage, green waste, Wastewater treatment, wastewater, and food waste. Biogas is produced by anaerobic ...
.
There are different methods of distributing the required components of combustion to a flame. In a diffusion flame, oxygen and fuel diffuse into each other; the flame occurs where they meet. In a premixed flame, the oxygen and fuel are premixed beforehand, which results in a different type of flame. Candle flames (a diffusion flame) operate through evaporation of the fuel which rises in a laminar flow of hot gas which then mixes with surrounding oxygen and combusts.
Color
 Flame color depends on several factors, the most important typically being
Flame color depends on several factors, the most important typically being black-body radiation
Black-body radiation is the thermal radiation, thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific ...
and spectral band emission, with both spectral line
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission (electromagnetic radiation), emission or absorption (electromagnetic radiation), absorption of light in a narrow frequency ...
emission and spectral line absorption playing smaller roles. In the most common type of flame, hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
flames, the most important factor determining color is oxygen supply and the extent of fuel-oxygen pre-mixing, which determines the rate of combustion and thus the temperature and reaction paths, thereby producing different color hues.
 In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about . The yellow arises from
In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about . The yellow arises from incandescence
Thermal radiation is electromagnetic radiation emitted by the thermal motion of particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation. The emission of energy arises from a combination of electron ...
of very fine soot particles that are produced in the flame. Also, carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
is produced, and the flame tends to take oxygen from the surfaces it touches. When the air inlet is opened, less soot and carbon monoxide are produced. When enough air is supplied, no soot or carbon monoxide is produced and the flame becomes blue. (Most of this blue had previously been obscured by the bright yellow emissions.) The spectrum of a premixed (complete combustion) butane
Butane () is an alkane with the formula C4H10. Butane exists as two isomers, ''n''-butane with connectivity and iso-butane with the formula . Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at ro ...
flame on the right shows that the blue color arises specifically due to emission of excited molecular radicals in the flame, which emit most of their light well below ≈565 nanometers in the blue and green regions of the visible spectrum.
The colder part of a diffusion (incomplete combustion) flame will be red, transitioning to orange, yellow, and white as the temperature increases as evidenced by changes in the black-body radiation spectrum. For a given flame's region, the closer to white on this scale, the hotter that section of the flame is. The transitions are often apparent in fires, in which the color emitted closest to the fuel is white, with an orange section above it, and reddish flames the highest of all. A blue-colored flame only emerges when the amount of soot decreases and the blue emissions from excited molecular radicals become dominant, though the blue can often be seen near the base of candles where airborne soot is less concentrated.
Specific colors can be imparted to the flame by introduction of excitable species with bright emission spectrum
The emission spectrum of a chemical element or chemical compound is the Spectrum (physical sciences), spectrum of frequencies of electromagnetic radiation emitted due to electrons making a atomic electron transition, transition from a high energ ...
lines. In analytical chemistry, this effect is used in flame tests (or flame emission spectroscopy) to determine presence of some metal ions. In pyrotechnics
Pyrotechnics is the science and craft of creating fireworks, but also includes safety matches, oxygen candles, Pyrotechnic fastener, explosive bolts (and other fasteners), parts of automotive airbags, as well as gas-pressure blasting in mining, q ...
, the pyrotechnic colorant
A pyrotechnic colorant is a chemical compound which causes a flame to burn with a particular color. These are used to create the colors in pyrotechnic compositions like fireworks and colored fires. The color-producing species are usually creat ...
s are used to produce brightly colored fireworks.
Temperature
 When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only an estimation of temperature. Other factors that determine its temperature are:
* In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion and the flame temperature is low, often just . This means that a lot of
When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only an estimation of temperature. Other factors that determine its temperature are:
* In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion and the flame temperature is low, often just . This means that a lot of carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
is formed (which is a flammable gas) which is when there is greatest risk of backdraft. When this occurs, combustible gases at or above the flash point of spontaneous combustion are exposed to oxygen, carbon monoxide and superheated hydrocarbons combust, and temporary temperatures of up to occur.
Common flame temperatures
This is a rough guide to flame temperatures for various common substances (in air at 1 atm. pressure):Highest temperature
Dicyanoacetylene, a compound of carbon and nitrogen with chemical formula C4N2 burns in oxygen with a bright blue-white flame at a temperature of , and at up to inozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
. This high flame temperature is partially due to the absence of hydrogen in the fuel (dicyanoacetylene is not a hydrocarbon) thus there is no water among the combustion products.
Cyanogen, with the formula (CN)2, produces the second-hottest-known natural flame with a temperature of over when it burns in oxygen.
Cool flames
At temperatures as low as , fuel-air mixtures can react chemically and produce very weak flames called cool flames. The phenomenon was discovered byHumphry Davy
Sir Humphry Davy, 1st Baronet (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several Chemical element, e ...
in 1817. The process depends on a fine balance of temperature and concentration of the reacting mixture, and if conditions are right it can initiate without any external ignition source. Cyclical variations in the balance of chemicals, particularly of intermediate products in the reaction, give oscillations in the flame, with a typical temperature variation of about , or between "cool" and full ignition. Sometimes the variation can lead to an explosion.
In microgravity
 In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on
In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on convection
Convection is single or Multiphase flow, multiphase fluid flow that occurs Spontaneous process, spontaneously through the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoy ...
, as soot tends to rise to the top of a flame (such as in a candle in normal gravity conditions), making it yellow. In microgravity
Weightlessness is the complete or near-complete absence of the sensation of weight, i.e., zero apparent weight. It is also termed zero g-force, or zero-g (named after the g-force) or, incorrectly, zero gravity.
Weight is a measurement of the fo ...
or zero gravity
Weightlessness is the complete or near-complete absence of the sensation of weight, i.e., zero apparent weight. It is also termed zero g-force, or zero-g (named after the g-force) or, incorrectly, zero gravity.
Weight is a measurement of the fo ...
environment, such as in orbit, natural convection no longer occurs and the flame becomes spherical, with a tendency to become bluer and more efficient. There are several possible explanations for this difference, of which the most likely is the hypothesis that the temperature is sufficiently evenly distributed that soot is not formed and complete combustion occurs. Experiments by NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the federal government of the United States, US federal government responsible for the United States ...
reveal that diffusion flames in microgravity allow more soot to be completely oxidized after they are produced than do diffusion flames on Earth, because of a series of mechanisms that behave differently in microgravity when compared to normal gravity conditions. These discoveries have potential applications in applied science
Applied science is the application of the scientific method and scientific knowledge to attain practical goals. It includes a broad range of disciplines, such as engineering and medicine. Applied science is often contrasted with basic science, ...
and private industry, especially concerning fuel efficiency
Fuel efficiency (or fuel economy) is a form of thermal efficiency, meaning the ratio of effort to result of a process that converts chemical energy, chemical potential energy contained in a carrier (fuel) into kinetic energy or Mechanical work, w ...
.
Edge flame
An edge flame or a triple flame refers to a stationary or moving flame edge in partially premixed reacting mixture. The canonical edge flame has a tribriachial structure that comprises two premixed flames, namely one fuel rich and one fuel lean, and a trailing diffusion flame. The theoretical development of triple flames was carried out by John W. Dold, Joel Daou and Amable Liñán.Thermonuclear flames
Flames do not need to be driven only by chemical energy release. In stars, subsonic burning fronts driven by burning light nuclei (like carbon or helium) to heavy nuclei (up to iron group) propagate as flames. This is important in some models of Type Ia supernovae. In thermonuclear flames, thermal conduction dominates over species diffusion, so the flame speed and thickness is determined by the thermonuclear energy release andthermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
(often in the form of degenerate electrons).
See also
* Flame detector * International Flame Research Foundation * Olympic flame * Oxidizing and reducing flames * The Combustion InstituteNotes
References
External links
A candle flame strongly influenced and moved about by an electric field due to the flame having ions.
(archived 30 September 2011)
Ultra-Low Emissions Low-Swirl Burner
7 Shades of Fire
(archived 31 August 2017) * {{Authority control Fire lez:ЦӀай