Emulsion stabilization using polyelectrolytes on:
[Wikipedia]
[Google]
[Amazon]
 When there is less
When there is less

 The general equation for repulsion energy assuming spherical particles (eq. 1):
:
where
: = particle radius,
: = bulk concentration of ions.
: =
The general equation for repulsion energy assuming spherical particles (eq. 1):
:
where
: = particle radius,
: = bulk concentration of ions.
: =
: = surface potential. Examples of polymers and their surface charge densities can be found in the table below.
Polyelectrolytes
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Ion#Anions and cations, Polycations and polyanions are polyelectrolytes. These groups dissociation (chemistry), dissociate in aqueous solutions (water), making the pol ...
are charged polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, b ...
capable of stabilizing (or destabilizing) colloidal emulsions through electrostatic interactions. Their effectiveness can be dependent on molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
, pH, solvent polarity, ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
, and the hydrophilic-lipophilic balance
The hydrophilic–lipophilic balance (HLB) of a surfactant is a measure of its degree of hydrophilicity or lipophilicity, determined by calculating percentages of molecular weights for the hydrophilic and lipophilic portions of the surfactant m ...
(HLB). Stabilized emulsions
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althoug ...
are useful in many industrial processes, including deflocculation, drug delivery, petroleum waste treatment, and food technology.
Types of polyelectrolytes
Polyelectrolytes are made up of positively or negatively chargedrepeat unit
A repeat unit or repeating unit , or mer, is a part of a polymer whose repetition would produce the complete polymer chain (except for the end groups) by linking the repeat units together successively along the chain, like the beads of a necklace ...
s. The charge
Charge or charged may refer to:
Arts, entertainment, and media Films
* ''Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* '' Charge!!'', an album by The Aqu ...
on a polyelectrolyte depends on the different properties of the solution, such as the degree of dissociation of the monomer units, the solvent properties, salt concentration, pH, and temperature.
Polymers become charged through the dissociation of the monomer side groups. If more monomer side groups are dissociated, the polymer has a higher charge. In turn, the charge
Charge or charged may refer to:
Arts, entertainment, and media Films
* ''Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* '' Charge!!'', an album by The Aqu ...
of the polymer classifies the polyelectrolyte, which can be positive (cationic) or negative (anionic).
The polymer charge and ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
of the polyelectrolyte in question dictate how thick a polyelectrolyte layer will be. The thickness of a polyelectrolyte then affects its adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
ability. For more information on polyelectrolyte adsorption, look here
Here may refer to:
Music
* ''Here'' (Adrian Belew album), 1994
* ''Here'' (Alicia Keys album), 2016
* ''Here'' (Cal Tjader album), 1979
* ''Here'' (Edward Sharpe album), 2012
* ''Here'' (Idina Menzel album), 2004
* ''Here'' (Merzbow album), ...
.
Some examples of polyelectrolytes can be found in the table below. The properties of the polymers vary with molecular weight and degree of polymerization.
Types of emulsions
The two main types ofemulsions
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althoug ...
are oil-in-water ( nonpolar in polar) and water-in-oil ( polar in nonpolar). The difference depends upon the nature of the surfactant
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a Blend word, blend of "surface-active agent",
coined in ...
or polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are t ...
in question. The hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
pieces will attract the polar solvent, creating a water-in-oil emulsion and the hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
pieces will attract the nonpolar solvent, creating an oil-in-water emulsion.
Emulsion stability
 When there is less
When there is less interfacial tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to ...
between the polyelectrolyte particles and the emulsions
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althoug ...
in question, emulsions are less stable. This is because the polyelectrolyte particles penetrate the flocs
In colloidal chemistry, flocculation is a process by which colloidal particles come out of suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipi ...
in suspension less when there is less interfacial tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to ...
.
Polyelectrolytes
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Ion#Anions and cations, Polycations and polyanions are polyelectrolytes. These groups dissociation (chemistry), dissociate in aqueous solutions (water), making the pol ...
adsorb
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
to the interface the emulsion
An emulsion is a mixture of two or more liquids that are normally Miscibility, immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloi ...
and help stabilize it, but may or may not lower the interfacial tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to ...
. This means that the oil or water droplets will not coalesce.
On their own, hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
surfactants cannot stabilize an emulsion
An emulsion is a mixture of two or more liquids that are normally Miscibility, immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloi ...
. Although they are attracted to oil, and an oil-in-water emulsion forms, the emulsion will not stay stable for long and will eventually coalesce. With the addition of a polyelectrolyte, electrostatic forces between the oil and water interface are formed and the surfactant begins to act as an “anchor” for the polyelectrolyte, stabilizing the emulsion. In addition to surfactants, nanoparticles can also help stabilize the emulsion by also providing a charged interface for the polyelectrolyte to adsorb on.
Molecular weight effects
The stability of the emulsion can depend on themolecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
of the accompanying polyelectrolyte. Polyelectrolytes of a high molecular weight are the most effective at stabilization. This is because they form a substantial steric barrier between oil and water, inhibiting aggregation. However, if the polyelectrolyte is too heavy it will not dissolve in the solution. Instead it will form gel lumps and fail to stabilize the emulsion.
pH effects
The effect of pH on thestability
Stability may refer to:
Mathematics
*Stability theory, the study of the stability of solutions to differential equations and dynamical systems
** Asymptotic stability
** Exponential stability
** Linear stability
**Lyapunov stability
** Marginal s ...
of polyelectrolytes
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Ion#Anions and cations, Polycations and polyanions are polyelectrolytes. These groups dissociation (chemistry), dissociate in aqueous solutions (water), making the pol ...
is based upon the functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
on the polymer backbone that is bearing the charge. A protonated amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
, for instance, will be much more stable at a lower pH while a sulfonate group will be more stable at a higher pH.
Solvent effects
Polyelectrolytes
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Ion#Anions and cations, Polycations and polyanions are polyelectrolytes. These groups dissociation (chemistry), dissociate in aqueous solutions (water), making the pol ...
will be much more soluble in polar solvents due to the charge on the polymer backbone and will spread out more. In nonpolar solvents, polyelectrolytes will coil becoming more densely packed and, if the backbone is nonpolar, will put the charge on the inside of the packed structure.
Ionic strength

Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
plays a crucial role in stability
Stability may refer to:
Mathematics
*Stability theory, the study of the stability of solutions to differential equations and dynamical systems
** Asymptotic stability
** Exponential stability
** Linear stability
**Lyapunov stability
** Marginal s ...
. In water-in-oil emulsions, as well as many others, the dielectric constant of the solvent is so low that the electrostatic forces between particles are not strong enough to have an effect on emulsion stability. Thus, emulsion stability depends greatly on the polyelectrolyte film thickness.
The polyelectrolyte film thickness is dependent upon its ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
. charged species on polyelectrolyte chains repel each other, causing the chains to stretch out. As the salt concentration increases, ionic strength increases, and the ions will shield the charges on the polymer chain allowing the polymer chain to form a dense random coil.
Theory
Electrostatic stabilization
Electrostatic repulsive forces dominate in polyelectrolyte stabilized emulsions., Although there are steric interactions, they are negligible in comparison. As theconcentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', ...
of polyelectrolyte increases, repulsive forces increase. When there are more polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are t ...
molecules, the distance between individual particles decreases. As the distance decreases, the exponential term becomes greater. Consequently, the repulsion energy also increases.
 The general equation for repulsion energy assuming spherical particles (eq. 1):
:
where
: = particle radius,
: = bulk concentration of ions.
: =
The general equation for repulsion energy assuming spherical particles (eq. 1):
:
where
: = particle radius,
: = bulk concentration of ions.
: = Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
,
: = reduced surface potential.
: = the surface to surface distance of the spherical particles.
: = the thermodynamic temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
: = the Debye length
In plasmas and electrolytes, the Debye length \lambda_\text (Debye radius or Debye–Hückel screening length), is a measure of a charge carrier's net electrostatic effect in a solution and how far its electrostatic effect persists. With each D ...
.
In addition, pH and ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
have a great influence on electrostatic interactions
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), meani ...
because these affect the "magnitude of electrical charge" in solution. As can be seen from the above equation, the repulsion energy depends on the square of the Debye length
In plasmas and electrolytes, the Debye length \lambda_\text (Debye radius or Debye–Hückel screening length), is a measure of a charge carrier's net electrostatic effect in a solution and how far its electrostatic effect persists. With each D ...
. From the equation for the Debye length
In plasmas and electrolytes, the Debye length \lambda_\text (Debye radius or Debye–Hückel screening length), is a measure of a charge carrier's net electrostatic effect in a solution and how far its electrostatic effect persists. With each D ...
, it is demonstrated how ionic strength can ultimately affect the electrostatic interactions in a solution.
Bjerrum length
Naturally, the question of the distance at which theseelectrostatic interactions
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), meani ...
become important arises. This can be discussed using the Bjerrum length. The Bjerrum length is the distance at which the electrostatic interaction between two charges is comparable to the thermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
, . The distance is given by eq. 2:
:
where
: = elementary charge
The elementary charge, usually denoted by , is a fundamental physical constant, defined as the electric charge carried by a single proton (+1 ''e'') or, equivalently, the magnitude of the negative electric charge carried by a single electron, ...
,
: = vacuum permittivity
Vacuum permittivity, commonly denoted (pronounced "epsilon nought" or "epsilon zero"), is the value of the absolute dielectric permittivity of classical vacuum. It may also be referred to as the permittivity of free space, the electric const ...
,
: = relative dielectric constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insul ...
.
Surface charge density
The factors discussed above can influence the charge on the surface of the polyelectrolyte. The surface charge density of these surfaces, at low surface potentials, can be modeled using a simplified version of the Grahame equation (eq. 3): where: = surface potential. Examples of polymers and their surface charge densities can be found in the table below.
Applications
Deflocculation
Depending on the situation,polyelectrolytes
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Ion#Anions and cations, Polycations and polyanions are polyelectrolytes. These groups dissociation (chemistry), dissociate in aqueous solutions (water), making the pol ...
can function as either flocculants or deflocculants. In order to stabilize emulsion, deflocculant polyelectrolytes are required.
When repulsive forces between particles overcome the intermolecular force
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
s in solution and the loose flocculated aggregates separate, deflocculation occurs. As opposed to the loose and easily separated sediments formed in flocculation, sediments formed in deflocculation are tightly packed and difficult to redisperse.
The repelling forces in a deflocculation increase the zeta potential
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.is a scientific term for Electrokinetic phenomena, electrokinetic Electric ...
, which in turn reduces the viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
of the suspension. Because of this reduction in viscosity, deflocculants are sometimes referred to as “thinning agents”. These thinning agents are usually alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The ...
and raise the pH of the suspension, preventing flocculation
In colloidal chemistry, flocculation is a process by which colloidal particles come out of Suspension (chemistry), suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The actio ...
. Deflocculants are used as thinning agents in molding plastics, making glassware, and creating clay ceramics.
Petroleum waste treatment
Polyelectrolytes
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Ion#Anions and cations, Polycations and polyanions are polyelectrolytes. These groups dissociation (chemistry), dissociate in aqueous solutions (water), making the pol ...
can also act as flocculants, separating solids (flakes) and liquids in industrial processes such as solubilization and oil recovery and they usually have a large cationic charge density
In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter ρ) is the quantity of charge per unit volume, measured in the SI system in co ...
.
Using organic materials to refine petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
instead of iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
or aluminum
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
coagulated would greatly decrease that amount of inorganic waste produced. The waste consists of stable oil-in-water emulsions. The addition of various polyelectrolytes to petroleum waste can cause the oil to coagulate, which will make it easier to remove and dispose of, and does not significantly decrease the stability of the solution.
Drug delivery

Polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are t ...
stabilized emulsions are important in the field of nanomedicine
Nanomedicine is the medical application of nanotechnology, translating historic nanoscience insights and inventions into practical application. Nanomedicine ranges from the medical applications of nanomaterials and biological devices, to n ...
. In order to function properly, any drug delivery system must be biocompatible and biodegradable
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
. Polyelectrolytes such as dextran sulfate (DSS), protamine (PRM) or poly-L-arginine all fulfill these requirements and may be used as a capsule with an emulsion
An emulsion is a mixture of two or more liquids that are normally Miscibility, immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloi ...
inside.
Oil in water emulsions are currently used as safe solvents
A solvent (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
for vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifi ...
s. It is important that these emulsion are stable
A stable is a building in which working animals are kept, especially horses or oxen. The building is usually divided into stalls, and may include storage for equipment and feed.
Styles
There are many different types of stables in use tod ...
and remain so for long periods of time. Polyelectrolyte stabilized emulsions could be used to increase the shelf life of vaccines. Researchers have been able to develop polyelectrolyte emulsions with more than six month stability.
In addition to being stable for extended periods of time, polyelectrolytes may be useful for vaccines because they can be biodegradable
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
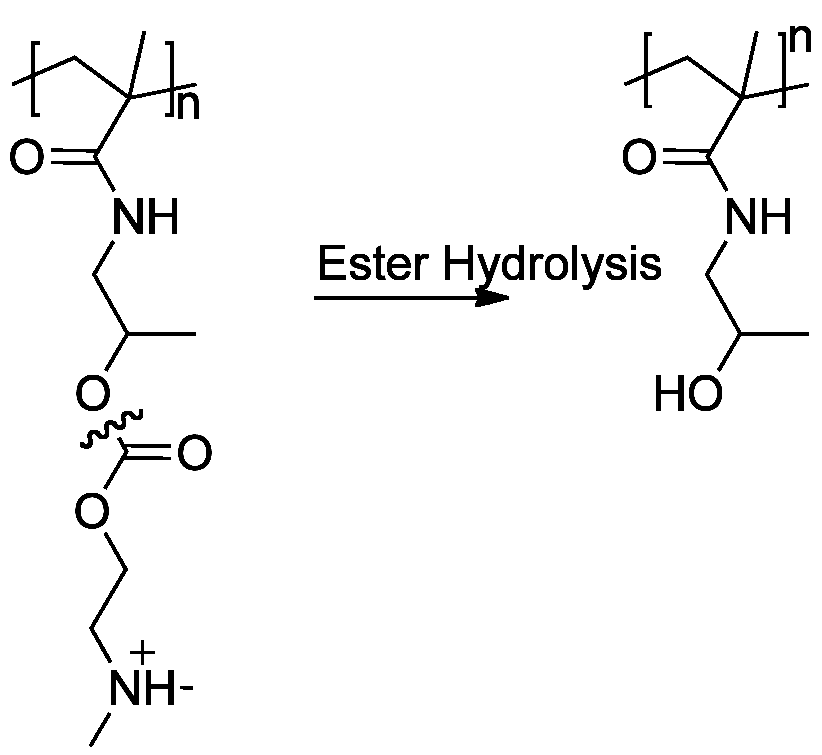
. For example, the ester bonds of the polyelectrolyte poly( HPMA- DMAE) can undergo hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
in the human body and VERO cells envelope DSS and use poly-L-arginine to break them down. Once the polylelectroyte capsule has been degraded, the emulsion containing drug is released into the body. Researchers have been investigating this drug delivery method to target leukemia cells.
Food technology
Because polyelectrolytes may be biocompatible, it follows that they can be used to stabilize emulsion in foods. Several studies have focused on usingpolyelectrolytes
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Ion#Anions and cations, Polycations and polyanions are polyelectrolytes. These groups dissociation (chemistry), dissociate in aqueous solutions (water), making the pol ...
to induce mixing of proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, re ...
and polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
in oil-in-water emulsions. DSS has been successfully used to stabilize these types of emulsions. Other studies have focused on stabilizing oil-in-water emulsions using β-lactoglobulin (β-Lg), a globular protein, and pectin
Pectin ( ': "congealed" and "curdled") is a heteropolysaccharide, a structural polymer contained in the primary lamella, in the middle lamella, and in the cell walls of terrestrial plants. The principal chemical component of pectin is galact ...
, an anionic polysaccharide. Both β-lactoglobulin and pectin are common ingredients in the food industry. β-lactoglobulin is used in whey protein, which can act as an emulsifier.
References
{{Reflist Chemical processes