Elution on:
[Wikipedia]
[Google]
[Amazon]
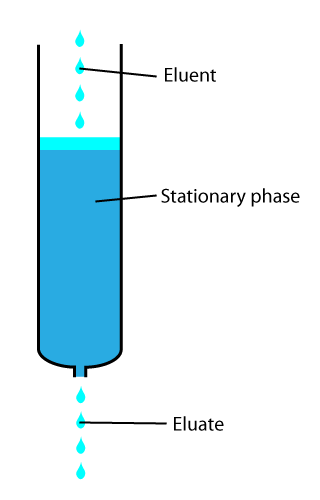 In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
In a liquid
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
In a liquid
Chemistry glossary
{{Authority control Analytical chemistry Chromatography
 In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
In a liquid
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
In a liquid chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
experiment, for example, an analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, et ...
is generally adsorbed, or "bound to", an adsorbent
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
in a liquid chromatography column. The adsorbent, a solid phase (stationary phase), is a powder which is coated onto a solid support. Based on an adsorbent's composition, it can have varying affinities
In post-classical history, an affinity was a collective name for the group ( retinue) of (usually) men whom a lord gathered around himself in his service; it has been described by one modern historian as "the servants, retainers, and other foll ...
to "hold" onto other molecules—forming a thin film on the surface of its particles. Elution then is the process of removing analytes from the adsorbent by running a solvent, called an "eluent", past the adsorbent/analyte complex. As the solvent molecules "elute", or travel down through the chromatography column, they can either pass by the adsorbent/analyte complex or they can displace the analyte by binding to the adsorbent in its place. After the solvent molecules displace the analyte, the analyte can be carried out of the column for analysis. This is why as the mobile phase passes out of the column, it typically flows into a detector
A sensor is a device that produces an output signal for the purpose of sensing a physical phenomenon.
In the broadest definition, a sensor is a device, module, machine, or subsystem that detects events or changes in its environment and sends ...
or is collected for compositional analysis.
Predicting and controlling the order of elution is a key aspect of column chromatographic methods.
Eluotropic series
An eluotropic series is listing of various compounds in order of eluting power for a givenadsorbent
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
. The "eluting power" of a solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
is largely a measure of how well the solvent can "pull" an analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, et ...
off the adsorbent to which it is attached. This often happens when the eluent adsorbs onto the stationary phase, displacing the analyte. Such series are useful for determining necessary solvents needed for chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
of chemical compounds. Normally such a series progresses from non-polar solvents, such as n-hexane, to polar solvents such as methanol or water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. The order of solvents in an eluotropic series depends both on the stationary phase as well as on the compound used to determine the order.
Eluent
The eluent or eluant is the "carrier" portion of the mobile phase. It moves the analytes through thechromatograph
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
. In liquid chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
, the eluent is the liquid solvent; in gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, ...
, it is the carrier gas.
Eluate
The eluate is theanalyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, et ...
material that emerges from the chromatograph
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
. It specifically includes both the analytes and solutes passing through the column, while the eluent is only the carrier.
Elution time and elution volume
The "elution time" of a solute is the time between the start of the separation (the time at which the solute enters the column) and the time at which the solute elutes. In the same way, the elution volume is the volume of eluent required to cause elution. Under standard conditions for a known mix of solutes in a certain technique, the elution volume may be enough information to identify solutes. For instance, a mixture ofamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s may be separated by ion-exchange chromatography. Under a particular set of conditions, the amino acids will elute in the same order and at the same elution volume.
Antibody elution
Antibody elution is the process of removing antibodies that are attached to their targets, such as the surface ofred blood cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
s. Techniques include using heat, a freeze/thaw cycle, ultrasound, acids or organic solvents. No single method is best in all situations.
See also
*Chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
* Desorption
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.
There ...
* Gradient elution in high performance liquid chromatography
* Leaching
References
*External links
Chemistry glossary
{{Authority control Analytical chemistry Chromatography