electron spectroscopy on:
[Wikipedia]
[Google]
[Amazon]
Electron spectroscopy refers to a group formed by techniques based on the analysis of the energies of emitted electrons such as photoelectrons and Auger electrons. This group includes
 As can be seen from the discussion above and figure 1, Auger electrons and photoelectrons are different in their physical origin, however, both types of electrons carry similar information about the chemical elements in material surfaces. Each element has its own special Auger electron or photon electron energy from which these can be identified. The binding energy of a photoelectron can be calculated by the formula below.
:
where ''Ebinding'' is the binding energy of the photoelectron, hν is the energy of the incoming radiation particle, ''Ekinetic'' is the kinetic energy of the photoelectron measured by the device and is the
As can be seen from the discussion above and figure 1, Auger electrons and photoelectrons are different in their physical origin, however, both types of electrons carry similar information about the chemical elements in material surfaces. Each element has its own special Auger electron or photon electron energy from which these can be identified. The binding energy of a photoelectron can be calculated by the formula below.
:
where ''Ebinding'' is the binding energy of the photoelectron, hν is the energy of the incoming radiation particle, ''Ekinetic'' is the kinetic energy of the photoelectron measured by the device and is the
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the very topmost 50-60 atoms, 5-10 nm of any surface. It belongs to the family of photoemission spectroscopies in which electro ...
(XPS), which also known as Electron Spectroscopy for Chemical Analysis (ESCA), Electron energy loss spectroscopy (EELS), Ultraviolet photoelectron spectroscopy
Ultraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra of photoelectrons emitted by molecules that have absorbed ultraviolet photons, in order to determine molecular orbital energies in the valence regio ...
(UPS), and Auger electron spectroscopy (AES). These analytical techniques are used to identify and determine the elements and their electronic structures from the surface of a test sample. Samples can be solids, gases or liquids.Yang Leng; ''Materials Characterization: Introduction to Microscopic and Spectroscopic Methods (Second Edition)''; Publisher John Wiley & Sons, Incorporated 2013; p: 191-192, 221-224.Daintith, J.; ''Dictionary of Chemistry (6th Edition)''; Oxford University Press, 2008; p: 191, 416, 541
Chemical information is obtained only from the uppermost atomic layers of the sample (depth 10 nm or less) because the energies of Auger electrons and photoelectrons are quite low, typically 20 - 2000 eV. For this reason, electron spectroscopy techniques are used to analyze surface chemicals.
History
The development of electron spectroscopy can be considered to have begun in 1887 when the German physicistHeinrich Rudolf Hertz
Heinrich Rudolf Hertz (; ; 22 February 1857 – 1 January 1894) was a German physicist who first conclusively proved the existence of the electromagnetic waves predicted by James Clerk Maxwell's equations of electromagnetism.
Biography
Heinric ...
discovered the photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
but was unable to explain it. In 1900, Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918.
Planck made many substantial con ...
(1918 Nobel Prize in Physics) suggested that energy carried by electromagnetic waves could only be released in "packets" of energy. In 1905 Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
(1921 Nobel Prize of Physics) explained Planck's discovery and the photoelectric effect. He presented the hypothesis that light energy is carried in discrete quantized packets (photons), each with energy E=hν to explain the experimental observations. Two years after this publication, in 1907, P. D. Innes recorded the first XPS spectrum.J. Theo Kloprogge, Barry J. Wood; ''Handbook of Mineral Spectroscopy: Volume 1: X-ray Photoelectron Spectra''; Elsevier 2020; p. xiii-xiv.
After numerous developments and the Second World War, Kai Siegbahn (Nobel Prize in 1981) with his research group in Uppsala, Sweden registered in 1954 the first XPS device to produce a high energy-resolution XPS spectrum. In 1967, Siegbahn published a comprehensive study of XPS and its usefulness, which he called electron spectroscopy for chemical analysis
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separa ...
(ESCA). Concurrently with Siegbahn's work, in 1962, David W. Turner at Imperial College London
Imperial College London, also known as Imperial, is a Public university, public research university in London, England. Its history began with Prince Albert of Saxe-Coburg and Gotha, Prince Albert, husband of Queen Victoria, who envisioned a Al ...
(and later Oxford University
The University of Oxford is a collegiate research university in Oxford, England. There is evidence of teaching as early as 1096, making it the oldest university in the English-speaking world and the second-oldest continuously operating u ...
) developed ultraviolet photoelectron spectroscopy
Ultraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra of photoelectrons emitted by molecules that have absorbed ultraviolet photons, in order to determine molecular orbital energies in the valence regio ...
(UPS) for molecular species using a helium lamp.
Basic theory
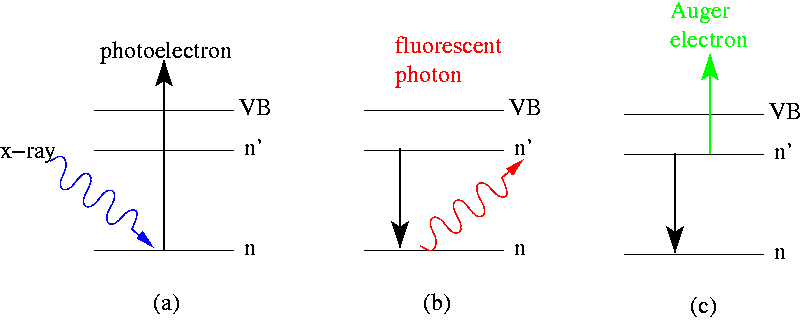
In electron spectroscopy, depending on the technique, irradiating the sample with high-energy particles such as X-ray photons, electron beam electrons, or ultraviolet radiation photons, causes Auger electrons and photoelectrons to be emitted. Figure 1 illustrates this on the basis of a single event in which, for example, an incoming X-ray photon from a particular energy range (E=hv) transfers its energy to an electron in the inner shell of an atom. The absorption of this photon ejects the electron and leaves a hole in the atomic shell (see figure 1 (a)). The hole can be filled in two ways, forming different characteristic rays that are specific to each element. If an electron from a shell with a higher energy level jumps to fill the hole, the energy difference can be emitted as a fluorescent photon (figure 1 (b)). In the Auger phenomenon, when the electron jumps from the higher energy level, its energy instead causes an adjacent or nearby electron to be ejected, forming an Auger electron (figure 1 (c)). As can be seen from the discussion above and figure 1, Auger electrons and photoelectrons are different in their physical origin, however, both types of electrons carry similar information about the chemical elements in material surfaces. Each element has its own special Auger electron or photon electron energy from which these can be identified. The binding energy of a photoelectron can be calculated by the formula below.
:
where ''Ebinding'' is the binding energy of the photoelectron, hν is the energy of the incoming radiation particle, ''Ekinetic'' is the kinetic energy of the photoelectron measured by the device and is the
As can be seen from the discussion above and figure 1, Auger electrons and photoelectrons are different in their physical origin, however, both types of electrons carry similar information about the chemical elements in material surfaces. Each element has its own special Auger electron or photon electron energy from which these can be identified. The binding energy of a photoelectron can be calculated by the formula below.
:
where ''Ebinding'' is the binding energy of the photoelectron, hν is the energy of the incoming radiation particle, ''Ekinetic'' is the kinetic energy of the photoelectron measured by the device and is the work function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
.
The kinetic energy of the Auger electron is approximately equal to the energy difference between the binding energies of the electron shells involved in the Auger process. This can be calculated as follows:
:
where ''Ekinetic'' is the kinetic energy of the Auger electron, hν is the energy of the incoming radiation particle and ''EB'' is first outer shell binding energy and ''EC'' is second outer shell binding energies.
Types of electron spectroscopy
*X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the very topmost 50-60 atoms, 5-10 nm of any surface. It belongs to the family of photoemission spectroscopies in which electro ...
* Auger electron spectroscopy
* Electron energy loss spectroscopy
* Ultraviolet photoelectron spectroscopy
Ultraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra of photoelectrons emitted by molecules that have absorbed ultraviolet photons, in order to determine molecular orbital energies in the valence regio ...
References
{{Authority control Spectroscopy