Dl-Alanine on:
[Wikipedia]
[Google]
[Amazon]
Alanine (symbol Ala or A), or α-alanine, is an α- amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its
 :
:
 Alanine is useful in loss of function experiments with respect to
Alanine is useful in loss of function experiments with respect to
IUPAC systematic name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for cho ...
is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
and does not need to be present in the diet. It is encoded by all codons
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
starting with GC (GCU, GCC, GCA, and GCG).
The L-isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
of alanine ( left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthes ...
in a sample of 1,150 proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
. The right-handed form, D-alanine, occurs in polypeptides in some bacterial cell wall
A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mech ...
s and in some peptide antibiotics, and occurs in the tissues of many crustaceans and molluscs as an osmolyte.
History and etymology
Alanine was first synthesized in 1850 when Adolph Strecker combinedacetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the mos ...
and ammonia with hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
. The amino acid was named '' Alanin'' in German, in reference to aldehyde, with the infix
An infix is an affix inserted inside a word stem (an existing word or the core of a family of words). It contrasts with ''adfix,'' a rare term for an affix attached to the outside of a stem, such as a prefix or suffix.
When marking text for int ...
''-an-'' for ease of pronunciation, the German ending '' -in'' used in chemical compounds being analogous to English ''-ine
''-ine'' is a suffix used in chemistry to denote two kinds of substance. The first is a chemically basic and alkaloidal substance. It was proposed by Joseph Louis Gay-Lussac in an editorial accompanying a paper by Friedrich Sertürner describing ...
''.
Structure
Alanine is an aliphatic amino acid, because the side-chain connected to theα-carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of ...
atom is a methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
group (-CH3); alanine is the simplest α-amino acid after glycine. The methyl side-chain of alanine is non-reactive and is therefore hardly ever directly involved in protein function. Alanine is a nonessential amino acid, meaning it can be manufactured by the human body, and does not need to be obtained through the diet. Alanine is found in a wide variety of foods, but is particularly concentrated in meats.
Sources
Biosynthesis
Alanine can be synthesized frompyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
and branched chain amino acids
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch (a central carbon atom bound to three or more carbon atoms). Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and v ...
such as valine, leucine, and isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprot ...
.
Alanine is produced by reductive amination of pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
, a two-step process. In the first step, α-ketoglutarate, ammonia and NADH are converted by glutamate dehydrogenase to glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
, NAD+ and water. In the second step, the amino group of the newly-formed glutamate is transferred to pyruvate by an aminotransferase enzyme, regenerating the α-ketoglutarate, and converting the pyruvate to alanine. The net result is that pyruvate and ammonia are converted to alanine, consuming one reducing equivalent. Because transamination reactions are readily reversible and pyruvate is present in all cells, alanine can be easily formed and thus has close links to metabolic pathways such as glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
, gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrat ...
, and the citric acid cycle.
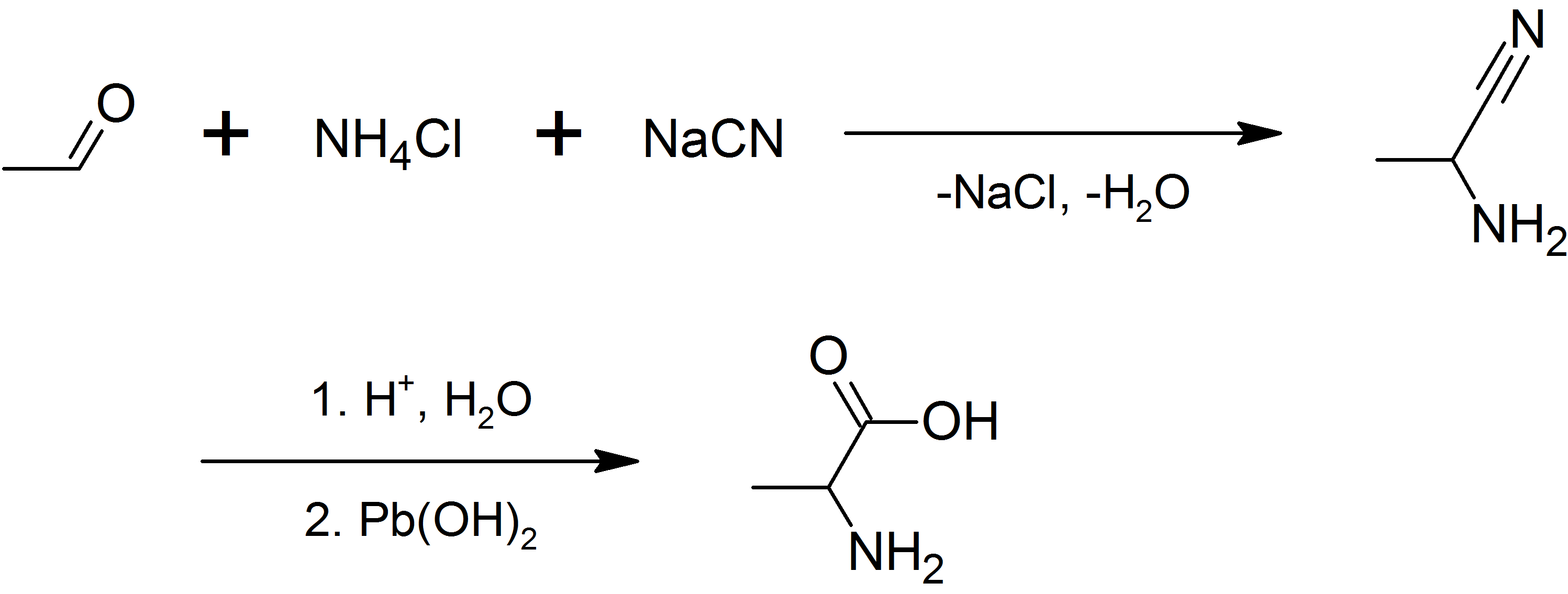
Chemical synthesis
L-Alanine is produced industrially by decarboxylation of L-aspartate by the action of aspartate 4-decarboxylase. Fermentation routes to L-alanine are complicated by alanine racemase. Racemic alanine can be prepared by the condensation ofacetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the mos ...
with ammonium chloride in the presence of sodium cyanide by the Strecker reaction, or by the ammonolysis of 2-bromopropanoic acid.
: :
:
Degradation
Alanine is broken down byoxidative deamination Oxidative deamination is a form of deamination that generates α-keto acids and other oxidized products from amine-containing compounds, and occurs primarily in the liver. Oxidative deamination is stereospecific, meaning it contains different stere ...
, the inverse reaction of the reductive amination reaction described above, catalyzed by the same enzymes. The direction of the process is largely controlled by the relative concentration of the substrates and products of the reactions involved.
Alanine World Hypothesis
Alanine is one of the twenty canonical α-amino acids used as building blocks (monomers) for the ribosome-mediated biosynthesis of proteins. Alanine is believed to be one of the earliest amino acids to be included in the genetic code standard repertoire. On the basis of this fact the "Alanine World" hypothesis was proposed. This hypothesis explains the evolutionary choice of amino acids in the repertoire of the genetic code from a chemical point of view. In this model the selection of monomers (i.e. amino acids) for ribosomal protein synthesis is rather limited to those Alanine derivatives that are suitable for building α-helix or β-sheetsecondary structural
Biomolecular structure is the intricate folded, three-dimensional shape that is formed by a molecule of protein, DNA, or RNA, and that is important to its function. The structure of these molecules may be considered at any of several length s ...
elements. Dominant secondary structures in life as we know it are α-helices and β-sheets and most canonical amino acids can be regarded as chemical derivatives of Alanine. Therefore, most canonical amino acids in proteins can be exchanged with Ala by point mutations while the secondary structure remains intact. The fact that Ala mimics the secondary structure preferences of the majority of the encoded amino acids is practically exploited in alanine scanning
In molecular biology, alanine scanning is a site-directed mutagenesis technique used to determine the contribution of a specific residue to the stability or function of a given protein. Alanine is used because of its non-bulky, chemically inert, ...
mutagenesis. In addition, classical X-ray crystallography often employs the polyalanine-backbone model to determine three-dimensional structures of proteins using molecular replacement
Molecular replacement (or MR) is a method of solving the phase problem in X-ray crystallography. MR relies upon the existence of a previously solved protein structure which is similar to our unknown structure from which the diffraction data is de ...
- a model-based phasing method.
Physiological function
Glucose–alanine cycle
In mammals, alanine plays a key role inglucose–alanine cycle
The Cahill cycle, also known as the alanine cycle or glucose-alanine cycle, is the series of reactions in which amino groups and carbons from muscle are transported to the liver. It is quite similar to the Cori cycle in the cycling of nutrients b ...
between tissues and liver. In muscle and other tissues that degrade amino acids for fuel, amino groups are collected in the form of glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
by transamination. Glutamate can then transfer its amino group to pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
, a product of muscle glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
, through the action of alanine aminotransferase, forming alanine and α-ketoglutarate. The alanine enters the bloodstream, and is transported to the liver. The alanine aminotransferase reaction takes place in reverse in the liver, where the regenerated pyruvate is used in gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrat ...
, forming glucose which returns to the muscles through the circulation system. Glutamate in the liver enters mitochondria
A mitochondrion (; ) is an organelle found in the Cell (biology), cells of most Eukaryotes, such as animals, plants and Fungus, fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosi ...
and is broken down by glutamate dehydrogenase into α-ketoglutarate and ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
, which in turn participates in the urea cycle to form urea which is excreted through the kidneys..
The glucose–alanine cycle enables pyruvate and glutamate to be removed from muscle and safely transported to the liver. Once there, pyruvate is used to regenerate glucose, after which the glucose returns to muscle to be metabolized for energy: this moves the energetic burden of gluconeogenesis to the liver instead of the muscle, and all available ATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
in the muscle can be devoted to muscle contraction. It is a catabolic pathway, and relies upon protein breakdown in the muscle tissue. Whether and to what extent it occurs in non-mammals is unclear.
Link to diabetes
Alterations in the alanine cycle that increase the levels of serum alanine aminotransferase (ALT) are linked to the development of type II diabetes.Chemical properties
 Alanine is useful in loss of function experiments with respect to
Alanine is useful in loss of function experiments with respect to phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
. Some techniques involve creating a library of genes, each of which has a point mutation at a different position in the area of interest, sometimes even every position in the whole gene: this is called "scanning mutagenesis". The simplest method, and the first to have been used, is so-called alanine scanning
In molecular biology, alanine scanning is a site-directed mutagenesis technique used to determine the contribution of a specific residue to the stability or function of a given protein. Alanine is used because of its non-bulky, chemically inert, ...
, where every position in turn is mutated to alanine.
Hydrogenation of alanine gives the amino alcohol alaninol
Alaninol is the organic compound with the formula CH3CH(NH2)CH2OH. A colorless solid, the compound is classified as an amino alcohol. It can be generated by converting the carboxylic group of alanine to an alcohol with a strong reducing agent s ...
, which is a useful chiral building block.
Free radical
The deamination of an alanine molecule produces the free radical CH3C•HCO2−. Deamination can be induced in solid or aqueous alanine by radiation that causes homolytic cleavage of the carbon–nitrogen bond.. This property of alanine is used in dosimetric measurements inradiotherapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radia ...
. When normal alanine is irradiated, the radiation causes certain alanine molecules to become free radicals, and, as these radicals are stable, the free radical content can later be measured by electron paramagnetic resonance in order to find out how much radiation the alanine was exposed to. This is considered to be a biologically relevant measure of the amount of radiation damage that living tissue would suffer under the same radiation exposure. Radiotherapy treatment plans can be delivered in test mode to alanine pellets, which can then be measured to check that the intended pattern of radiation dose is correctly delivered by the treatment system.
References
{{Authority control Proteinogenic amino acids Glucogenic amino acids Glycine receptor agonists NMDA receptor agonists