|
Aminotransferase
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α- keto acid. They are important in the synthesis of amino acids, which form proteins. Function and mechanism An amino acid contains an amino (NH2) group. A keto acid contains a keto (=O) group. In transamination, the NH2 group on one molecule is exchanged with the =O group on the other molecule. The amino acid becomes a keto acid, and the keto acid becomes an amino acid. Transaminases require the coenzyme pyridoxal phosphate, which is converted into pyridoxamine in the first half-reaction, when an amino acid is converted into a keto acid. Enzyme-bound pyridoxamine in turn reacts with pyruvate, oxaloacetate, or alpha-ketoglutarate, giving alanine, aspartic acid, or glutamic acid, respectively. Many transamination reactions occur in tissues, catalysed by transaminases specific for a particular amino/keto acid pair. The reactions are readily reversible, the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate Transaminase
Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase (GOT, SGOT), is a pyridoxal phosphate (PLP)-dependent transaminase enzyme () that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder. Serum AST level, serum ALT (alanine transaminase) level, and their ratio (AST/ALT ratio) are commonly measured clinically as biomarkers for liver health. The tests are part of blood test, blood panels. The biological half-life, half-life of total AST in the circulation approximates 17 hours and, on average, 87 hours for ''mitochondrial'' AST. Aminotransferase is cleared by Liver sinusoidal endothelial cell, sinusoidal cells in the liver. Function Aspartate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids.This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids (amino acids that can be synthesized de novo by the organism). Transamination in biochemistry is accomplished by enzymes called transaminases or aminotransferases. α-ketoglutarate acts as the predominant amino-group acceptor and produces glutamate as the new amino acid. :Amino acid, Aminoacid + α-ketoglutarate ↔ α-keto acid + Glutamic acid, glutamate Glutamate's amino group, in turn, is transferred to oxaloacetate in a second transamination reaction yielding aspartate. :Glutamic acid, Glutamate + oxaloacetate ↔ α-ketoglutarate + aspartate Mechanism of action Transamination catalyzed by aminotransferase occurs in two stages. In the first step, the α amino group of an amino acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridoxal Phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates. Role as a coenzyme PLP acts as a coenzyme in all transamination reactions, and in certain decarboxylation, deamination, and racemization reactions of amino acids. The aldehyde group of PLP forms a Schiff-base linkage (internal aldimine) with the ε-amino group of a specific lysine group of the aminotransferase enzyme. The α-amino group of the amino acid substrate displaces the ε-amino group of the active-site lysine residue in a process known as transaldimination. The r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
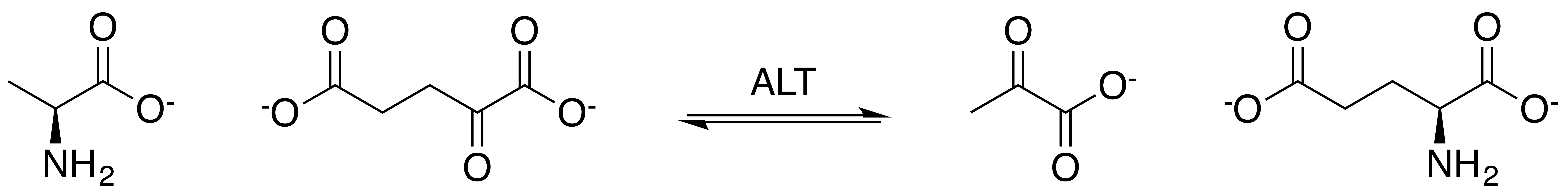
Alanine Transaminase
Alanine aminotransferase (ALT or ALAT), formerly alanine transaminase (ALT), and even earlier referred to as serum glutamate-pyruvate transaminase (GPT) or serum glutamic-pyruvic transaminase (SGPT), is a transaminase enzyme () that was first characterized in the mid-1950s by Arthur Karmen and colleagues. ALT is found in plasma and in various body tissues but is most common in the liver. It catalyzes the two parts of the alanine cycle. Serum ALT level, serum AST ( aspartate transaminase) level, and their ratio ( AST/ALT ratio) are routinely measured clinically as biomarkers for liver health. The half-life of ALT in the circulation approximates 47 hours. Aminotransferase is cleared by sinusoidal cells in the liver. Function ALT catalyzes the transfer of an amino group from L-alanine to α-ketoglutarate, the products of this reversible transamination reaction being pyruvate and L-glutamate. :L-alanine + α-ketoglutarate ⇌ pyruvate + L-glutamate ALT (and all aminotran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently it is classified as a non-polar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as ) and its carboxyl group deprotonated (as ). It is non-essential to humans as it can be synthesized metabolically and does not need to be present in the diet. It is encoded by all codons starting with G C (GC U, GCC, GC A, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to L-leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in peptides in some bacterial cell walls (in peptidoglycan) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elevated Transaminases
In medicine, the presence of elevated transaminases, commonly the transaminases alanine transaminase (ALT) and aspartate transaminase (AST), may be an indicator of liver dysfunction. Other terms include transaminasemia, and elevated liver enzymes (though they are not the only enzymes in the liver). Normal ranges for both ALT and AST vary by gender, age, and geography and are roughly 8-40 U/L (0.14-0.67 μkal/L). Mild transaminesemia refers to levels up to 250 U/L. Drug-induced increases such as that found with the use of anti-tuberculosis agents such as isoniazid are limited typically to below 100 U/L for either ALT or AST. Muscle sources of the enzymes, such as intense exercise, are unrelated to liver function and can markedly increase AST and ALT. Cirrhosis of the liver or fulminant liver failure secondary to hepatitis commonly reach values for both ALT and AST in the >1000 U/L range; however, many people with liver disease have normal transaminases. Elevated transaminases t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine Cycle
The Cahill cycle, also known as the alanine cycle or glucose-alanine cycle, is the series of reactions in which amino groups and carbons from muscle are transported to the liver. It is quite similar to the Cori cycle in the cycling of nutrients between skeletal muscle and the liver. When muscles degrade amino acids for energy needs, the resulting nitrogen is transaminated to pyruvate to form alanine. This is performed by the enzyme alanine transaminase (ALT), which converts L-Glutamic acid, glutamate and pyruvate into α-ketoglutarate and L-alanine. The resulting L-alanine is shuttled to the liver where the nitrogen enters the urea cycle and the pyruvate is used to make glucose. The Cahill cycle is less productive than the Cori cycle, which uses lactate, since a byproduct of energy production from alanine is production of urea. Removal of the urea is energy-dependent, requiring four "high-energy" phosphate bonds (3 Adenosine triphosphate, ATP hydrolyzed to 2 Adenosine diphosphate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons. Its molecular formula is . Glutamic acid exists in two optically isomeric forms; the optical rotation, dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxylic acid, carboxyl groups −COOH and one amine, amino group � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of proteins. D-aspartic acid is one of two D-amino acids commonly found in mammals. Apart from a few rare exceptions, D-aspartic acid is not used for protein synthesis but is incorporated into some peptides and plays a role as a neurotransmitter/ neuromodulator. Like all other amino acids, aspartic acid contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of various proteins and various other Biochemistry, biochemicals necessary for digestion and growth. In humans, it is located in the quadrants and regions of abdomen, right upper quadrant of the abdomen, below the thoracic diaphragm, diaphragm and mostly shielded by the lower right rib cage. Its other metabolic roles include carbohydrate metabolism, the production of a number of hormones, conversion and storage of nutrients such as glucose and glycogen, and the decomposition of red blood cells. Anatomical and medical terminology often use the prefix List of medical roots, suffixes and prefixes#H, ''hepat-'' from ἡπατο-, from the Greek language, Greek word for liver, such as hepatology, and hepatitis The liver is also an accessory digestive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamic Acid
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α- amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons. Its molecular formula is . Glutamic acid exists in two optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartic Acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of proteins. D-aspartic acid is one of two D-amino acids commonly found in mammals. Apart from a few rare exceptions, D-aspartic acid is not used for protein synthesis but is incorporated into some peptides and plays a role as a neurotransmitter/ neuromodulator. Like all other amino acids, aspartic acid contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |