Dithiolene on:
[Wikipedia]
[Google]
[Amazon]
Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species, are unsaturated bidentate
 Because of the unusual redox and intense optical properties of dithiolenes, the electronic structure of dithiolene complexes has been the subject of intense studies. 1,2-Dithiolene ligands can exist in three
Because of the unusual redox and intense optical properties of dithiolenes, the electronic structure of dithiolene complexes has been the subject of intense studies. 1,2-Dithiolene ligands can exist in three 
 1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of conductivity,
1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of conductivity,

ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
wherein the two donor atoms are sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
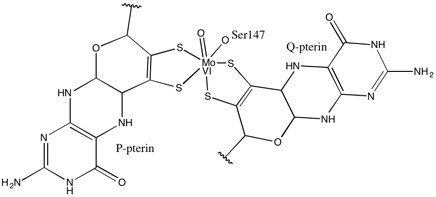
. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin
Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopteri ...
cofactor bound to the Mo or W.
Dithiolene metal complexes have been studied since the 1960s when they were first popularized by Gerhard N. Schrauzer and Volker P. Mayweg, who prepared nickel bis(stilbene-1,2-dithiolate) (Ni(S2C2Ph2)2) by the reaction of nickel sulfide
Nickel sulfide is any inorganic compound with the formula NiSx. They range in color from bronze (Ni3S2) to black (NiS2). The nickel sulfide with simplest stoichiometry is NiS, also known as the mineral millerite. From the economic perspective, ...
and diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.
Prepa ...
. The structural, spectroscopic, and electrochemical properties of many related complexes have been described.
Structure and bonding
Dithiolene metal complexes can be found in coordination compounds where the metal centre is coordinated by one, two, or three dithiolene ligands. The tris(dithiolene) complexes were the first examples of trigonal prismatic geometry in coordination chemistry. One example is Mo(S2C2Ph2)3. Similar structures have been observed for several other metals. Because of the unusual redox and intense optical properties of dithiolenes, the electronic structure of dithiolene complexes has been the subject of intense studies. 1,2-Dithiolene ligands can exist in three
Because of the unusual redox and intense optical properties of dithiolenes, the electronic structure of dithiolene complexes has been the subject of intense studies. 1,2-Dithiolene ligands can exist in three oxidation states
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. ...
: the dianionic "ene-1,2-dithiolate", the neutral "1,2-dithioketone," and a monoanionic radical intermediate between these two. When the latter two are complexed to a metal centre, the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. ...
of the ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
(and therefore the metal centre) cannot be easily defined. Such ligands are therefore referred to as non-innocent. The substituents on the backbone of the dithiolene ligand, R and R', affect the properties of the resulting metal complex in the expected way. Long chains confer solubility in less polar solvents. Electron acceptors (e.g. CN, CO2Me) stabilize reduced and anionic complexes. Derivatives are known where the substituents are the same, symmetrical dithiolenes (R = R') are more common than unsymmetrical.
Due to their delocalized electronic structure, 1,2-dithiolene complexes undergo reversible redox reaction. When oxidized, dithiolene complexes have greater 1,2-dithioketone character. In reduced complexes, the ligand assumes more ene-1,2-dithiolate character. These descriptions are evaluated by examination of differences in C-C and C-S bond distances. The true structure lies somewhere between these resonance structures. Reflecting the impossibility to provide an unequivocal description of the structure, McCleverty introduced the term 'dithiolene' to give a general name for the ligand that does not specify a particular oxidation state. This suggestion was generally accepted, and 'dithiolene' is now a universally accepted term. Only more recently the radical nature of monoanionic 1,2-dithiolene ligands has been pointed out. While few examples of authentic dithiolene radicals have been reported, diamagnetism in neutral bis(1,2-dithiolene) complexes of divalent transition metal ions should be considered as a consequence of a string antiferromagnetic coupling between the two radical ligands.
:
Applications and occurrence
1,2-Dithiolene metal complexes occur widely in nature in the form of the molybdopterin-bound Mo and W-containing enzymes. 1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of conductivity,
1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of conductivity, magnetism
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particle ...
, and nonlinear optics
Nonlinear optics (NLO) is the branch of optics that describes the behaviour of light in ''nonlinear media'', that is, media in which the polarization density P responds non-linearly to the electric field E of the light. The non-linearity is typic ...
. It was proposed to use dithiolene metal complexes that bind unsaturated hydrocarbons at the sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
centers for industrial olefin (alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
) purifications. However, the complexities within such systems became later apparent, and it was argued that more research would be needed before using metal dithiolene complexes in alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
purifications may become practical.
Preparation
From alkenedithiolates
Most dithiolene complexes are prepared by reaction of alkali metal salts of 1,2-alkenedithiolates with metal halides. A thiolate is the conjugate base of athiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
, so alkenedithiolate is, formally speaking, the conjugate base of an alkenedithiol. Common alkenedithiolates are 1,3-dithiole-2-thione-4,5-dithiolate and maleonitriledithiolate (mnt2−):
: Ni2+ + 2 (NC)2C2S22− → Ni 2C2(CN)2sub>22−
Some alkenedithiolates are generated in situ, often by complex organic reactions:
: cis-H2C2(SCH2Ph)2 + 4 Na → cis-H2C2(SNa)2 + 2 NaCH2Ph
Once generated, these anions are deployed as ligands:
: NiCl2 + 2 cis-H2C2(SNa)2 → Na2 i(S2C2H2)2 + 2 NaCl
Often the initially formed, electron-rich complex undergoes spontaneous air-oxidation:
: i(S2C2H2)2sup>2− + 2 H+ + 1/2 O2 → Ni(S2C2H2)2 + H2O

From acyloins
An early and still powerful method for the synthesis of dithiolenes entails the reaction of α-hydroxyketones,acyloin
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl gro ...
s, with P4S10 followed by hydrolysis and treatment of the mixture with metal salts. This method is used to prepare Ni 2C2Ar2sub>2 (Ar = aryl).
From dithietes
Although 1,2-dithiones are rare and thus not useful precursors, theirvalence isomer In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.
Benzene
There are many valence isomers one can draw for the C6H6 formula benzene. Some were originall ...
, the 1,2-dithiete
Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp2-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of ...
s are occasionally used. One of the more common dithiete is the distillable (CF3)2C2S2, prepared from the reaction of elemental sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
and hexafluoro-2-butyne
Hexafluoro-2-butyne (HFB) is a fluorocarbon with the chemical structure CF3C≡CCF3. HFB is a particularly electrophilic acetylene derivative, and hence a potent dienophile for Diels–Alder reactions.
HFB is prepared by the action of sulfu ...
. This electrophilic reagent oxidatively adds to many low valent metals to give bis- and tris(dithiolene) complexes.
: Mo(CO)6 + 3 (CF3)2C2S2 → CF3)2C2S2sub>3Mo + 6 CO
: Ni(CO)4 + 2 (CF3)2C2S2 → CF3)2C2S2sub>2Ni + 4 CO
By reactions of metal sulfides with alkynes
Species of the type Ni 2C2Ar2sub>2 were first prepared by reactions of nickel sulfides withdiphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.
Prepa ...
. More modern versions of this method entail the reaction of electrophilic acetylenes such as dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate (DMAD) is an organic compound with the formula CH3O2CC2CO2CH3. It is a di-ester in which the ester groups are conjugated with a C-C triple bond. As such, the molecule is highly electrophilic, and is widely employed ...
with well defined polysulfido complexes.
History and nomenclature
Early studies on dithiolene ligands, although not called by that name until the 1960s, focused on the quinoxaline-2,3-dithiolates and 3,4-toluenedithiol
Benzene-1,2-dithiol is the organosulfur compound with the formula CH(SH). This colourless viscous liquid consists of a benzene ring with a pair of adjacent thiol groups. The conjugate base of this diprotic compound serves as chelating agent in coo ...
ates, which form brightly colored precipitates with several metal centres. Such species were originally of interest in analytical chemistry. Dithiolenes lacking benzene backbones represented an important development of the area, especially maleonitrile-1,2-dithiolate ("mnt"), (NC)2C2S22−, and ethylenedithiolene, H2C2S22−.
References
{{Coordination complexes Coordination complexes Thiols Chelating agents Coordination chemistry