|
Hexafluoro-2-butyne
Hexafluoro-2-butyne (HFB) is a fluorocarbon with the chemical structure CF3C≡CCF3. HFB is a particularly electrophilic alkyne, acetylene derivative (chemistry), derivative, and hence a potent dienophile for Diels–Alder reactions. Synthesis and reactions HFB is prepared by the action of sulfur tetrafluoride on acetylenedicarboxylic acid or by the reaction of potassium fluoride (KF) with hexachlorobutadiene. In the presence of the strong Lewis acid aluminium chlorofluoride, hexafluorobutadiene isomerizes to HFB: : HFB reacts with sulfur to give 3,4-Bis(trifluoromethyl)-1,2-dithiete, 3,4-bis(trifluoromethyl)-1,2-dithiete. Cycloaddition of HFB and Dithionitronium hexafluoroarsenate, dithionitronium (NS2+) gives the 1,2,5-dithiazolium cation. This derivative can be reduced to the 7 electron neutral radical. This particular 1,3,5-dithiazole is also rare example of a radical that can be obtained as solid, liquid, and gaseous states. As a gas, it is blue. As an electrophilic alk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, meaning they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are also called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye
The debye ( , ; symbol: D) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is given as charge times length of separation. The dipole itself is a vector whose direction coincides with the position vector of the positive charge with respect to the negative charge: : p = ''q''r. named in honour of the physicist Peter J. W. Debye. It is defined as statcoulomb-centimetres.The statcoulomb is also known as the franklin or electrostatic unit of charge. : 1 statC = 1 Fr = 1 esu = 1 cm3/2⋅g1/2⋅s−1. Historically the debye was defined as the dipole moment resulting from two charges of opposite sign but an equal magnitude of 10−10 statcoulomb10−10 statcoulomb corresponds to approximately 0.2083 units of elementary charge. (generally called e.s.u. (electrostatic unit) in ol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective. Preparation Potassium fluoride is prepared by reacting potassium carbonate with hydrofluoric acid. Evaporation of the solution forms crystals of potassium bifluoride. The bifluoride on heating yields potassium fluoride: : : Platinum or heat resistant plastic containers are often used for these operations. Potassium chloride converts to KF upon treatment with hydrogen fluoride. In this way, potassium fluoride is recyclable. Crystalline properties KF crystallizes in the cubic NaCl crystal structure. The lattice parameter at room temperature is 0.266 nm. Applications in organic chemistry In organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne Derivatives
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiazolium
Dithiazolium refers to families of heterocycles consisting of C2NS2 rings. The cations are aromatic on the grounds that they have six pi-electrons. In principle, several isomers are possible, depending on the relative location of the C, N, and S atoms in the ring. 1,2,5-Dithiazolium cations arise from cycloaddition of dithionitronium (NS2+) to alkynes. The derivative with two trifluoromethyl groups (prepared from hexafluorobutyne) can be reduced to the 7 electron radical. This particular 1,3,5-dithiazole is also rare example of a radical that can be obtained as a solid, liquid, and gas. The gas is blue. Benzo-1,2-3-dithiazolium salts can be prepared by the Herz reaction, which entails the reaction of an aniline with disulfur dichloride. Hydrolysis of this "Herz salt" gives the corresponding sodium thiolate Sodium hydrosulfide is the chemical compound with the formula NaSH. This compound is the product of the half-neutralization of hydrogen sulfide () with sodium hydroxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithionitronium Hexafluoroarsenate
Dithionitronium hexafloroarsenate is the inorganic compound with the formula . It is the hexafluoroarsenate () salt of S=N=S+. The cation is of interest as the sulfur analogue of nitronium (). Hexafloroarsenate is a weakly coordinating anion. According to X-ray crystallography, S=N=S+ is linear with S-N distances of 146 picometers. Synthesis and reactions Dithionitronium hexafluoroarsenate is prepared from thiazyl chloride using silver hexafluoroarsenate. The hexachloroantimonate salt can be prepared by treating thiazyl chloride with sulfur in the presence of antimony pentachloride according to this idealized equation: : The dithionitronium cation reacts with nitriles to give dithiadiazolium salts: : Addition to alkynes gives dithiazolium Dithiazolium refers to families of heterocycles consisting of C2NS2 rings. The cations are aromatic on the grounds that they have six pi-electrons. In principle, several isomers are possible, depending on the relative location of the C, N, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,4-Bis(trifluoromethyl)-1,2-dithiete
3,4-Bis(trifluoromethyl)-1,2-dithiete is the organofluorine compound with the formula , a yellow liquid. It is a stable 1,2-dithiete. It arises by the reaction of hexafluoro-2-butyne with molten sulfur. Bonding Being planar with six pi-electrons, the compound is considered to be aromatic. This description is supported by an electron diffraction study, which reveals an elongated C=C distance of 1.40 Å and shortened C-S distances of 1.73 Å. Reactions The compound tends to dimerize at room temperature, but the dimer cracks at higher temperature back to the dithiete. It is used to prepare metal dithiolene complexes. It reacts with low valent metal complexes by oxidative addition Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...: : : References {{DEFAULTSORT:Bis(trifluoromethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorobutadiene
Hexafluorobutadiene is an organofluorine compound Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, ... with the formula (CF2=CF)2. A colorless gas, it has attracted attention as an etchant in microelectronics. It is the perfluoroanalogue of butadiene. Preparation It can be prepared by coupling of fluorinated C2 precursors. Addition of iodine monochloride to chlorotrifluoroethylene gives iododichlorotrifluoroethane that can be coupled in the presence of mercury to give 1,2,3,4-tetrchlorohexafluorobutane: : Zn-induced dechlorination of this tetrachloride gives the desired perfluorinated diene: : Reactions The diene can be rehalogenated, e.g. with bromine upon UV irradiation: : Hexafluorobutadiene dimerizes via a [2+2] process at 150 °C to give perfluorinated divinylcyclobutanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane CH3)3Bis a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexachlorobutadiene
Hexachlorobutadiene, (often abbreviated as "HCBD") Cl2C=C(Cl)C(Cl)=CCl2, is a colorless liquid at room temperature that has an odor similar to that of turpentine. It is a chlorinated aliphatic diene with niche applications but is most commonly used as a solvent for other chlorine-containing compounds.Manfred Rossberg et al. "Chlorinated Hydrocarbons," Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co, 2006, Kenric A. Marshall, "Chlorocarbons and Chlorohydrocarbons, Survey," Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons Inc, 2003, Structurally, it has a 1,3-butadiene core, but fully substituted with chlorine atoms. Synthesis Hexachlorobutadiene is primarily produced in chlorinolysis plants as a by-product in the production of carbon tetrachloride and tetrachloroethene. Chlorinolysis is a radical chain reaction that occurs when hydrocarbons are exposed to chlorine gas under pyrolytic conditions. The hydrocarbon is chlorinated and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
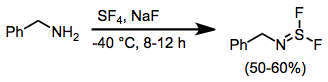
Sulfur Tetrafluoride
Sulfur tetrafluoride is a chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the +4 oxidation state, with one lone pair of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |