Dicyan on:
[Wikipedia]
[Google]
[Amazon]
Cyanogen is the
 Paracyanogen is a
Paracyanogen is a
National Pollutant Inventory - Cyanide compounds fact sheet
{{Authority control Alkanedinitriles Blood agents Lachrymatory agents Pseudohalogens
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. Its structure is . The simplest stable carbon nitride
Carbon nitrides are organic compounds consisting only of carbon and nitrogen atoms.
Covalent network compounds
These materials are organic semiconductors. Due to its hydrogen-bonding motifs and electron-rich properties, this carbon material is ...
, it is a colorless and highly toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
gas
Gas is a state of matter that has neither a fixed volume nor a fixed shape and is a compressible fluid. A ''pure gas'' is made up of individual atoms (e.g. a noble gas like neon) or molecules of either a single type of atom ( elements such as ...
with a pungent
Pungency ( ) is the taste of food commonly referred to as spiciness, hotness or heat, found in foods such as chili peppers. Highly pungent tastes may be experienced as unpleasant. The term piquancy ( ) is sometimes applied to foods with a lower ...
odor
An odor (American English) or odour ( Commonwealth English; see spelling differences) is a smell or a scent caused by one or more volatilized chemical compounds generally found in low concentrations that humans and many animals can perceive ...
. The molecule is a pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
. Cyanogen molecules are linear
In mathematics, the term ''linear'' is used in two distinct senses for two different properties:
* linearity of a '' function'' (or '' mapping'');
* linearity of a '' polynomial''.
An example of a linear function is the function defined by f(x) ...
, and consist of two CN groups ‒ analogous to diatomic halogen molecules, such as Cl, but far less oxidizing. The two cyano groups are bonded together at their carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms, though other isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide
Cyanogen bromide is the inorganic compound with the chemical formula, formula BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compo ...
() (but see also ''Cyano radical
The cyano radical (or cyanido radical) is a radical with molecular formula CN, sometimes written •CN. The cyano radical was one of the first detected molecules in the interstellar medium, in 1938. Its detection and analysis was influential in as ...
''). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene
Dicyanoacetylene, also called carbon subnitride or but-2-ynedinitrile (IUPAC), is a compound of carbon and nitrogen with chemical formula . At room temperature, dicyanoacetylene is a colorless volatile liquid. It has a linear molecular structure ...
).
Cyanogen is the anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid (chemistry), acid.
In organic chemistry, organic acid anhydrides contain the functional group . Organic acid anhydrides often form when one ...
of oxamide
Oxamide is the organic compound with the formula . This white crystalline solid is soluble in ethanol, slightly soluble in water and insoluble in diethyl ether. Oxamide is the diamide derived from oxalic acid, and the hydrate of cyanogen.
Prepa ...
:
:
though oxamide is manufactured from cyanogen by hydrolysis:
:
Preparation
Cyanogen is typically generated fromcyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
compounds. One laboratory method entails thermal decomposition of mercuric cyanide
Mercury(II) cyanide, also known as mercuric cyanide, is a poisonous compound of mercury and cyanide. It is an odorless, toxic white powder. It is highly soluble in polar solvents such as water, alcohol, and ammonia, slightly soluble in ether, an ...
:
:
Or, one can combine solutions of copper(II) salts (such as copper(II) sulfate
Copper(II) sulfate is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, whi ...
) with cyanides; an unstable copper(II) cyanide is formed which rapidly decomposes into copper(I) cyanide
Copper(I) cyanide (cuprous cyanide) is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in ele ...
and cyanogen.
:
Industrially, it is created by the oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
, usually using chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
over an activated silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
or nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, is an intermediate in the s ...
over a copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
salt. It is also formed when nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
are reacted by an electrical spark or discharge.
Reactions
For the two less stable isomers of cyanogen, the order of the atoms differs. Isocyanogen (or cyanogen cyanide) is . It has been detected in theinterstellar medium
The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ...
.
Addition of disulfur dichloride
Disulfur dichloride (or disulphur dichloride by the British English spelling) is the inorganic compound of sulfur and chlorine with the Chemical formula, formula . It is an amber oily liquid.
Sometimes, this compound is incorrectly named ''sulfur ...
to cyanogen gives 3,4-dichloro-1,2,5-thiadiazole
3,4-Dichloro-1,2,5-thiadiazole is the heterocycle with the formula . It is a colorless liquid. The compound is produced by treating cyanogen with disulfur dichloride. The compound can also be prepared from aminoacetonitrile.
It is a planar mol ...
.
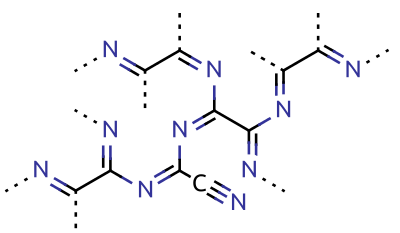
Paracyanogen
 Paracyanogen is a
Paracyanogen is a polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
of cyanogen. It can be best prepared by heating mercury(II) cyanide
Mercury(II) cyanide, also known as mercuric cyanide, is a poisonous compound of mercury and cyanide. It is an odorless, toxic white powder. It is highly soluble in polar solvents such as water, alcohol, and ammonia, slightly soluble in ether ...
. It can also be prepared by heating silver cyanide
Silver cyanide is the chemical compound with the formula AgCN. It is a white salt that is precipitated upon treatment of solutions containing Ag+ with cyanide, which is used in some schemes to recover silver from solution. Silver cyanide is used ...
, silver cyanate
Silver cyanate is the cyanate salt of silver. It can be made by the reaction of potassium cyanate with silver nitrate in aqueous solution, from which it precipitates as a solid.
:
Alternatively, the reaction
:
analogous to the reaction used for ...
, cyanogen iodide
Cyanogen iodide or iodine cyanide is a compound with the chemical formula . It is a pseudohalogen composed of iodine and the cyanide group. It is a highly toxic inorganic compound. It occurs as white crystals that react slowly with water to form h ...
or cyanuric iodide. It can also be prepared by the polymerization of cyanogen at in the presence of trace impurities. Paracyanogen can also be converted back to cyanogen by heating to . Based on experimental evidence, the structure of this polymeric material is thought to be rather irregular, with most of the carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms being of sp type and localized domains of π conjugation.
History
Cyanogen was first synthesized in 1815 byJoseph Louis Gay-Lussac
Joseph Louis Gay-Lussac ( , ; ; 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for his discovery that water is made of two parts hydrogen and one part oxygen by volume (with Alexander von Humboldt), f ...
, who determined its empirical formula and named it. Gay-Lussac coined the word "cyanogène" from the Greek words κυανός (kyanos, blue) and γεννάω (gennao, to create), because cyanide was first isolated by Swedish chemist Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish Pomerania, German-Swedish pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified the elements molybd ...
from the pigment Prussian blue
Prussian blue (also known as Berlin blue, Brandenburg blue, Parisian and Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula . It consists of cations, where iron is in the oxidat ...
. It attained importance with the growth of the fertilizer
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Man ...
industry in the late 19th century and remains an important intermediate in the production of many fertilizers. It is also used as a stabilizer in the production of nitrocellulose
Nitrocellulose (also known as cellulose nitrate, flash paper, flash cotton, guncotton, pyroxylin and flash string, depending on form) is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and ...
.
Cyanogen is commonly found in comet
A comet is an icy, small Solar System body that warms and begins to release gases when passing close to the Sun, a process called outgassing. This produces an extended, gravitationally unbound atmosphere or Coma (cometary), coma surrounding ...
s. In 1910 a spectroscopic
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectrosc ...
analysis of Halley's Comet
Halley's Comet is the only known List of periodic comets, short-period comet that is consistently visible to the naked eye from Earth, appearing every 72–80 years, though with the majority of recorded apparitions (25 of 30) occurring after ...
found cyanogen in the comet's tail, which led to public fear that the Earth would be poisoned as it passed through the tail. People in New York
New York most commonly refers to:
* New York (state), a state in the northeastern United States
* New York City, the most populous city in the United States, located in the state of New York
New York may also refer to:
Places United Kingdom
* ...
wore gas masks, and merchants sold quack
Quack, The Quack or Quacks may refer to:
People
* Quack Davis, American baseball player
* Hendrick Peter Godfried Quack (1834–1917), Dutch economist and historian
* Joachim Friedrich Quack (born 1966), German Egyptologist
* Johannes Quack ...
"comet pills" claimed to neutralize poisoning. Because of the extremely diffuse nature of the tail, there was no effect when the planet passed through it.
Safety
Like othercyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
s, cyanogen is very toxic, as it readily undergoes reduction to cyanide, which poisons the cytochrome c oxidase
The enzyme cytochrome c oxidase or Complex IV (was , now reclassified as a translocasEC 7.1.1.9 is a large transmembrane protein complex found in bacteria, archaea, and the mitochondria of eukaryotes.
It is the last enzyme in the Cellular respir ...
complex, thus interrupting the mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
l electron transfer chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this ...
. Cyanogen gas is an irritant to the eyes and respiratory system. Inhalation can lead to headache, dizziness, rapid pulse, nausea, vomiting, loss of consciousness, convulsions, and death, depending on exposure. Lethal dose through inhalation typically ranges from .
Cyanogen produces the second-hottest-known natural flame (after dicyanoacetylene
Dicyanoacetylene, also called carbon subnitride or but-2-ynedinitrile (IUPAC), is a compound of carbon and nitrogen with chemical formula . At room temperature, dicyanoacetylene is a colorless volatile liquid. It has a linear molecular structure ...
aka carbon subnitride) with a temperature of over when it burns in oxygen.
In popular culture
In the ''Doctor Who
''Doctor Who'' is a British science fiction television series broadcast by the BBC since 1963. The series, created by Sydney Newman, C. E. Webber and Donald Wilson (writer and producer), Donald Wilson, depicts the adventures of an extraterre ...
'' serial "The Brain of Morbius
''The Brain of Morbius'' is the fifth serial of the Doctor Who (season 13), 13th season of the British science fiction television series ''Doctor Who'', which was first broadcast in four weekly parts on BBC One, BBC1 from 3 to 24 January 1976. Th ...
" (the 5th serial of season 13), the Doctor synthesizes cyanogen using hydrogen cyanide as a starting material and vents it through a pipe to stop Solon from performing surgery on the brain of Morbius's body.
In '' Dragnet'' (1987) Friday (Dan Aykroyd) and Streebek (Tom Hanks) are tracking down the villain who stole "the pseudohalogenic compound cyanogen".
In the second season of '' The Night Agent'', cyanogen is stolen from a chemical plant as part of a plot to manufacture a chemical weapon
A chemical weapon (CW) is a specialized munition that uses chemicals formulated to inflict death or harm on humans. According to the Organisation for the Prohibition of Chemical Weapons (OPCW), this can be any chemical compound intended as ...
.
See also
*Pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
References
External links
* *National Pollutant Inventory - Cyanide compounds fact sheet
{{Authority control Alkanedinitriles Blood agents Lachrymatory agents Pseudohalogens