|
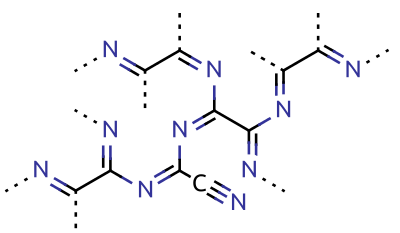
Carbon Nitride
Carbon nitrides are organic compounds consisting only of carbon and nitrogen atoms. Covalent network compounds These materials are organic semiconductors. Due to its hydrogen-bonding motifs and electron-rich properties, this carbon material is considered a potential candidate for material applications in carbon supplementation. * Beta carbon nitride - a solid with a formula , which is predicted to be harder than diamond. * Graphitic carbon nitride - , with important catalytic and sensor properties. * Dicyanodiazomethane , the only isomer studied experimentally * Tricyanamide - monomer (has never been prepared yet) * Dicyanocarbodiimide - another monomer (was detected in products of photolysis of triazido-s-triazine). * - a combined triazole and triazine framework. * MCN-12 () and MCN-13 (). Azafullerenes * Azafullerenes are a class of heterofullerenes in which the element substituting for carbon is nitrogen. Examples include (biazafullerenyl),Hummelen et al, "Is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicyanoacetylene
Dicyanoacetylene, also called carbon subnitride or but-2-ynedinitrile (IUPAC), is a compound of carbon and nitrogen with chemical formula . At room temperature, dicyanoacetylene is a colorless volatile liquid. It has a linear molecular structure, (often abbreviated as ), with alternating triple and single covalent bonds. It can be viewed as acetylene with the two hydrogen atoms replaced by cyanide groups. Because of its high endothermic heat of formation, dicyanoacetylene can explode to carbon powder and nitrogen gas, : and it burns in oxygen with a bright blue-white flame at a temperature of , the hottest flame in oxygen; burned in ozone at high pressure the flame temperature exceeds . Dicyanoacetylene polymerizes at room temperature into a dark solid. Synthesis Dicyanoacetylene can be prepared by passing nitrogen gas over a sample of graphite heated to temperatures between . It may also be synthesized via a reaction between a dihaloacetylene and a cyanide salt: : As a r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazole
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing Heterocyclic compound, heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-membered ring, that exists in two equivalent tautomeric f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazine
Tetrazine is a compound that consists of a six-membered aromatic ring containing four nitrogen atoms with the molecular formula C2 H2 N4. The name ''tetrazine'' is used in the nomenclature of derivatives of this compound. Three core-ring isomers exist: 1,2,3,4-tetrazines, 1,2,3,5-tetrazines, and 1,2,4,5-tetrazines, also known as v-tetrazines, as-tetrazines and s-tetrazines respectively. 1,2,3,4-Tetrazines 1,2,3,4-Tetrazines are often isolated fused to an aromatic ring system and are stabilized as the dioxide derivatives. 1,2,4,5-Tetrazines 1,2,4,5-Tetrazines are very well known and myriad 3,6-disubstituted 1,2,4,5-tetrazines are known. These materials are of use in the area of energetic chemistry. Heavily substituted tetrazines form the verdazyls, a family of stable radicals. Protected tetrazines are strong acetylene acceptors in Diels-Alder equilibria. For example, dipyridinyl 1,2,4,5-tetrazine abstracts acetylene from norbornadiene to cyclopentadiene and a pyrida ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazine
Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is . They exist in three isomeric forms, 1,3,5-triazines being common. Structure The triazines have planar six-membered benzene-like ring but with three carbons replaced by nitrogens. The three isomers of triazine are distinguished by the positions of their nitrogen atoms, and are referred to as 1,2,3-triazine, 1,2,4-triazine, and 1,3,5-triazine. Other aromatic nitrogen heterocycles are pyridines with one ring nitrogen atom, diazines with 2 nitrogen atoms in the ring, triazoles with 3 nitrogens in a 5-membered ring, and tetrazines with 4 ring nitrogen atoms. Uses Melamine A well known triazine is melamine (2,4,6-triamino-1,3,5-triazine). With three amino substituents, melamine is a precursor to commercial resins. Guanamines are closely related to melamine, except with one amino substituent replaced by an organic group. This difference is exploited in the use of guanamine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a ''"deliquescent crystal or wax-like solid with a pungent, sweet, corn-like, nutty odour"''. Pyrazine and a variety of alkylpyrazines are flavor and aroma compounds found in baked and roasted goods. Tetramethylpyrazine (also known as ligustrazine) is reported to scavenge superoxide anions and decrease nitric oxide production in human granulocytes. Synthesis Many methods exist for the organic synthesis of pyrazine and its derivatives. Some of these are among the oldest synthesis reactions still in use. In the Staedel–Rugheimer pyrazine synthesis (1876), 2-chloroacetophenone is reacted with ammonia to the amino ketone, then condensed and then oxidized to a pyrazine. A variation is the Gutknecht pyrazine synthesis (1879) also based on this selfcondensation, but di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated Polymer, polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties Pyridine is diamagnetism, diamagnetic. Its critical point (thermodynamics), critical parameters are: pressure 5.63 MPa, temperature 619 K and volume 248 cm3/mol. In the temperatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraazidomethane
Tetraazidomethane, , is a colorless, highly explosive liquid. Its chemical structure consists of a carbon atom covalently bonded to four azide functional groups. Synthesis It was first prepared by Klaus Banert in 2006 by reaction of trichloroacetonitrile with sodium azide."The Exciting Chemistry of Tetraazidomethane", Klaus Banert, Young-Hyuk Joo, Tobias Ruffer, Bernhard Walfort, and Heinrich Lang, ''Angew. Chem. Int. Ed.'' 2007, 46, 1168–1171. : Uses As with other polyazides, tetraazidomethane has interest as a high-energy-density material with potential uses in explosives, propellants, or fireworks."Tetraazidomethane: Chemistry with a Bang", ''Chemical & Engineering News'', Dec. 18, 2006, 46. Silicon tetraazide is also a known compound. Reactions Banert has reported that tetraazidomethane participates in a number of reactions including hydrolysis, cycloaddition reactions with alkenes and alkynes, and reaction with phosphines to form phosphazenes. : References {{Az ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen
Cyanogen is the chemical compound with the chemical formula, formula . Its structure is . The simplest stable carbon nitride, it is a Transparency and translucency, colorless and highly toxic gas with a pungency, pungent odor. The molecule is a pseudohalogen. Cyanogen molecules are linear molecular geometry, linear, and consist of two CN groups ‒ analogous to diatomic halogen molecules, such as chlorine, Cl, but far less oxidizing. The two cyanide, cyano groups are bonded together at their carbon atoms, though other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide () (but see also ''Cyano radical''). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene). Cyanogen is the anhy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyyne
A polyyne is any organic compound with alternating Single bond, single and triple bonds; that is, a series of consecutive alkynes, with ''n'' greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds with ''n'' greater than 1. They are also sometimes referred to as oligoynes, or carbinoids after "linear acetylenic carbon, carbyne" , the hypothetical allotrope of carbon that would be the ultimate member of the series. In ''Avancés récentes en chimie des acétylènes – Recent advances in acetylene chemistry'' The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today. The simplest polyyne is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, single bonds or double bond, double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as diphosphorus and carbon monoxide, are also triple bonded. In skeletal formula, skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding Triple bonding can be explained in terms of orbital hybridization. In the case of acetylene, each carbon atom has two sp orbital, sp-orbitals and two p-orbitals. The two sp-orbitals are linear, with 180° bond angles, and occupy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single Bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of the two electrons involved is no longer in the sole possession of the Atomic orbital, orbital in which it originated. Rather, both of the two electrons spend time in either of the orbitals which overlap in the bonding process. As a Lewis structure, a single bond is denoted as AːA or A-A, for which A represents an element. In the first rendition, each dot represents a shared electron, and in the second rendition, the bar represents both of the electrons shared in the single bond. A covalent bond can also be a double bond or a triple bond. A single bond is weaker than either a double bond or a triple bond. This difference in strength can be explained by examining the component bonds of which each of these types of covalent bonds consists (Moo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |