diazomethane on:
[Wikipedia]
[Google]
[Amazon]
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow
 In more specialized applications, diazomethane and other diazoalkyl reagents are used in the Arndt–Eistert reaction and the Büchner–Curtius–Schlotterbeck reaction for
In more specialized applications, diazomethane and other diazoalkyl reagents are used in the Arndt–Eistert reaction and the Büchner–Curtius–Schlotterbeck reaction for  Diazomethane reacts with
Diazomethane reacts with
 Diazomethane reacts with
Diazomethane reacts with
MSDS diazomethaneSigmaaldrich technical bulletin
(PDF)
diazomethane applications and commercial availability of (Diazald) precursor
Identification of Artifacts (By-Products) in Diazomethane and Trimethylsilyldiazomethane ReactionsA flask of diazomethane solution, photographed
{{Nitrogen compounds Diazo compounds Methylating agents IARC Group 3 carcinogens Reagents for organic chemistry Explosive chemicals 1894 in science Gases with color Explosive gases Organic compounds with 1 carbon atom
gas
Gas is a state of matter that has neither a fixed volume nor a fixed shape and is a compressible fluid. A ''pure gas'' is made up of individual atoms (e.g. a noble gas like neon) or molecules of either a single type of atom ( elements such as ...
; thus, it is almost universally used as a solution in diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.
Use
For safety and convenience diazomethane is always prepared as needed as a solution inether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
and used as such. It converts carboxylic acids to methyl esters and phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ar ...
into their methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give a methyldiazonium cation, which reacts with the carboxylate ion to give the methyl ester and nitrogen gas. Labeling studies indicate that the initial proton transfer is faster than the methyl transfer step. Since proton transfer is required for the reaction to proceed, this reaction is selective for the more acidic carboxylic acids (p''K''a ~ 5) and phenols (p''K''a ~ 10) over aliphatic alcohols (p''K''a ~ 15).
 In more specialized applications, diazomethane and other diazoalkyl reagents are used in the Arndt–Eistert reaction and the Büchner–Curtius–Schlotterbeck reaction for
In more specialized applications, diazomethane and other diazoalkyl reagents are used in the Arndt–Eistert reaction and the Büchner–Curtius–Schlotterbeck reaction for homologation
Homologation (Greek language, Greek ''homologeo'', ὁμολογέω, "to agree") is the granting of approval by an official authority. This may be a court of law, a government department, or an academic or professional body, any of which would n ...
of various compounds.
alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s or phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
s in presence of boron trifluoride
Boron trifluoride is the inorganic compound with the formula . This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bonding
The g ...
(BF3) to give methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s.
Diazomethane is also frequently used as a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
source. It readily takes part in 1,3-dipolar cycloadditions.
Preparation
Laboratory scale
A wide variety of routes have been developed for the laboratory production of diazomethane. In general, the synthesis of these all involves the addition ofmethylamine
Methylamine, also known as methanamine, is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.
Methylamine is sold ...
to an electron-deficient species, before treatment with nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
and mineral acid (nitrous acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite () salts. It was discovered by Carl Wilhelm Scheele, who called it " phlogisticated acid of niter". Nitrous ac ...
) to form an ''N''-methyl nitrosamide. Diazomethane is prepared by hydrolysis of an ethereal solution of these ''N''-methyl nitrosamides with aqueous base. Examples include:
* ''N''-nitroso-''N''-methylurea (NMU), the original precursor first reported by Hans von Pechmann in 1894 and historically one of the most popular choices. Its popularity has slowly waned due to it being unstable at above 20 °C and somewhat shock-sensitive.
* ''N''-Nitroso-β-methylaminoisobutyl methyl ketone (Liquizald), another early precursor which remains in use in the present day.
* ''N'',''N''-dimethyl-''N'',''N''-dinitrosoterephthalamide (DMDMT)
* ''N''-methyl-''N-nitro-''N''-nitrosoguanidine (MNNG), used as both a biochemical tool and a diazomethane source.
* ''N''-methyl-''N''-nitroso-''p''-toluenesulfonamide (Diazald), one of the most popular modern precursors.
: Diazomethane reacts with
Diazomethane reacts with alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The ...
solutions of D2O to give the deuterated derivative CD2N2. This can be used for isotopic labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through chemical reaction, metabolic pathway, or a biological cell. The reactant is 'labeled' ...
studies.
Industrial use
The ease with which diazomethane explodes makes it too hazardous to handle in large quantities. Despite this, it can be used on an industrial scale using on-demand flow chemistry. In these processes the rate of production is matched by the rate of consumption, such that the amount of diazomethane present at any one time is very low.Analysis
The concentration of CH2N2 can be determined in either of two convenient ways. It can be treated with an excess ofbenzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
in cold Et2O. Unreacted benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
is then back-titrated with standard NaOH. Alternatively, the concentration of CH2N2 in Et2O can be determined spectrophotometrically at 410 nm where its extinction coefficient, ε, is 7.2.
The gas-phase concentration of diazomethane can be determined using photoacoustic spectroscopy.
Related compounds
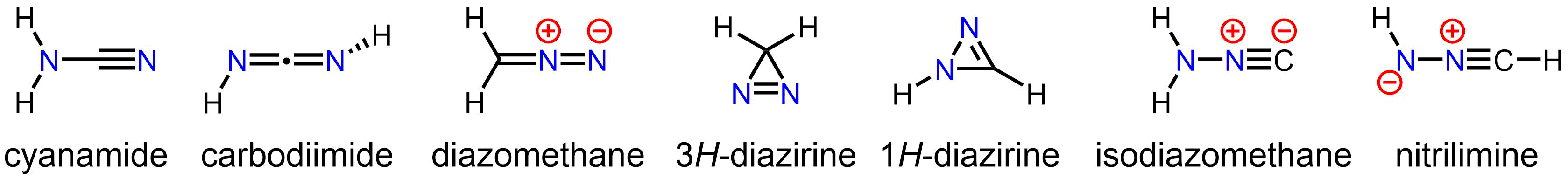
Diazomethane is both isomeric andisoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
with the more stable cyanamide
Cyanamide is an organic compound with the formula C N2 H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a ...
, but they do not interconvert.
Many substituted derivatives of diazomethane have been prepared:
*The very stable (CF3)2CN2 (2-diazo-1,1,1,3,3,3-hexafluoropropane; b.p. 12–13 °C),
*Ph2CN2 ( diazodiphenylmethane; m.p. 29–30 °C).
*(CH3)3SiCHN2 ( trimethylsilyldiazomethane), which is commercially available as a solution and is as effective as CH2N2 for methylation.
* PhC(H)N2, a red liquid b.p.< 25 °C at 0.1 mmHg.
Safety
Diazomethane is toxic by inhalation or by contact with the skin or eyes (TLV 0.2 ppm). Symptoms include chest discomfort, headache, weakness and, in severe cases, collapse.Muir, GD (ed.) 1971, ''Hazards in the Chemical Laboratory'', The Royal Institute of Chemistry, London. Symptoms may be delayed. Deaths from diazomethane poisoning have been reported. In one instance a laboratory worker consumed a hamburger near a fumehood where he was generating a large quantity of diazomethane, and died four days later from fulminating pneumonia.LeWinn, E.B. "Diazomethane Poisoning: Report of a fatal case with autopsy", ''The American Journal of the Medical Sciences'', 1949, 218, 556-562. Like any otheralkylating agent Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
it is expected to be carcinogenic, but such concerns are overshadowed by its serious acute toxicity.
CH2N2 may explode in contact with sharp edges, such as ground-glass joints, even scratches in glassware. Glassware should be inspected before use and preparation should take place behind a blast shield. Specialized kits to prepare diazomethane with flame-polished joints are commercially available.
The compound explodes when heated beyond 100 °C, exposed to intense light, alkali metals, or calcium sulfate. Use of a blast shield is highly recommended while using this compound.
Proof-of-concept work has been done with microfluidics
Microfluidics refers to a system that manipulates a small amount of fluids (10−9 to 10−18 liters) using small channels with sizes of ten to hundreds of micrometres. It is a multidisciplinary field that involves molecular analysis, molecular bi ...
, in which continuous point-of-use synthesis from ''N''-methyl-''N''-nitrosourea and 0.93 M potassium hydroxide in water was followed by point-of-use conversion with benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
, resulting in a 65% yield of the methyl benzoate ester within seconds at temperatures ranging from 0 to 50 °C. The yield was better than under capillary conditions; the microfluidics were credited with "suppression of hot spots, low holdup, isothermal conditions, and intensive mixing."
Isomers
The stable compoundcyanamide
Cyanamide is an organic compound with the formula C N2 H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a ...
, whose minor tautomer is carbodiimide, is an isomer of diazomethane. Less stable but still isolable isomers of diazomethane include the cyclic 3''H''-diazirine and isocyanoamine ( isodiazomethane). In addition, the parent nitrilimine Nitrilimines or nitrile amides are a class of organic compounds sharing a common functional group with the general structure corresponding to the conjugate base of an amine bonded to the N-terminus of a nitrile. The dominant structure for the paren ...
has been observed under matrix isolation conditions.

References
External links
MSDS diazomethane
(PDF)
diazomethane applications and commercial availability of (Diazald) precursor
Identification of Artifacts (By-Products) in Diazomethane and Trimethylsilyldiazomethane Reactions
{{Nitrogen compounds Diazo compounds Methylating agents IARC Group 3 carcinogens Reagents for organic chemistry Explosive chemicals 1894 in science Gases with color Explosive gases Organic compounds with 1 carbon atom