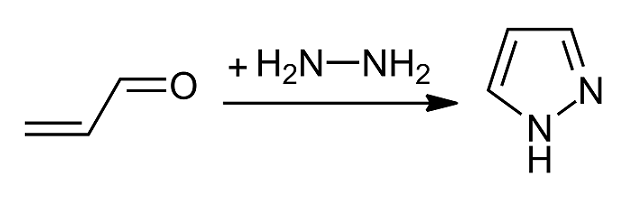|
Nitrilimine
Nitrilimines or nitrile amides are a class of organic compounds sharing a common functional group with the general structure corresponding to the conjugate base of an amine bonded to the N-terminus of a nitrile. The dominant structure for the parent compound nitrilimine is that of the propargyl-like in ''scheme 1'' with a C–N triple bond and with a formal positive charge on nitrogen and two lone pairs and a formal negative charge on the terminal nitrogen. Other structures such as hypervalent , allene-like , allylic and carbene are of lesser relevance. Nitrilimines were first observed in the thermal decomposition of 2-tetrazoles releasing nitrogen: Nitrilimines are linear 1,3-dipoles represented by structures and . A major use is in heterocyclic synthesis. E.g. with alkynes they generate pyrazoles in a 1,3-dipolar cycloaddition. Due to their high energy, they are usually generated ''in situ'' as a reactive intermediate. References [Baidu] |
Tetrazole
A tetrazole is a chemical synthesis, synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound - a whitish crystalline powder with the formula CH2N4, of which three isomers exist. Structure and bonding Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1''H''-, 2''H''-, and 5''H''-tetrazole. The 1''H''- and 2''H''- isomers are tautomers, with the equilibrium lying on the side of 1''H''-tetrazole in the solid phase. In the gas phase, 2''H''-tetrazole dominates. These isomers can be regarded as aromatic, with 6 π-electrons, while the 5''H''-isomer is nonaromatic. Phosphorus analogs do not have the same electronic nature, with 1''H''-tetraphosphole having a more pyramidal geometry of the phosphorus at position 1. Instead, it is the anionic tetraphospholides that are aromatic. Strongly inductive effect, inductively electron-withd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms. They are reactants in 1,3-dipolar cycloadditions. The dipole has at least one resonance structure with positive and negative charges having a 1,3 relationship which can generally be denoted as , where a may be a carbon, oxygen or nitrogen, b may be nitrogen or oxygen, and c may be a carbon, oxygen or nitrogen. Known 1,3-dipoles are: * Azides () * Ozone () * Nitro compounds () * Diazo compounds () * Some oxides ** Azoxide compounds (RN(O)NR) ** Carbonyl oxides ( Criegee zwitterions)Li, Jie Jack''Criegee mechanism of ozonolysis''Book: Name Reactions. 2006, 173-174, ** Nitrile oxides () ** Nitrous oxide () ** Nitrones () * Some imines: ** Azomethine imine ** Nitrilimines (, analogous to nitrile oxide) ** Carbonyl imines * Some ylide An ylide () or ylid () is a neutral dipolar molecule containing a formally negatively c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene radical, methylene, the parent hydride from which all other carbene compounds are formally derived. There are two types of carbenes: singlet state, singlets or triplet state, triplets, depending upon their electronic structure. The different classes undergo different reactions. Most carbenes are extremely reactive and short-lived. A small number (the diHalogen, halocarbenes, carbon monoxide, and carbon monosulfide) can be isolated, and can stabilize as Coordination complex, metal ligands, but otherwise cannot be stored in bulk. A rare exception are the persistent carbenes, which have extensive application in modern organometallic chemistry. Generatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazole
Pyrazole is an organic compound with the chemical formula, formula . It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in Arene substitution pattern, ortho-substitution. Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Properties Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å History The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883. In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition of a substance caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Since thermal decomposition is a kinetic process, the observed temperature of its beginning in most instances will be a function of the experimental conditions and sensitivity of the experimental setup. For a rigorous depiction of the process, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reactions that tend to occur with al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |