Copper-free Click Chemistry on:
[Wikipedia]
[Google]
[Amazon]
Copper-free click chemistry is a bioorthogonal reaction as a variant of an
azide-alkyne Huisgen cycloaddition
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Ka ...
. By eliminating cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are toxic metals, toxic chemicals, microbe neurotoxins, radiation particles and even specific neurotransmitters when the system is out of balance. Also some types of dr ...
copper catalysts, the reaction proceeds without live-cell toxicity. It was developed as a faster alternative to the Staudinger ligation with the first generation of Cu-free click chemistry, producing rate constants over 63 times faster.
Although the reaction produces a regioisomeric mixture of triazoles, the lack of regioselectivity in the reaction is not a major concern for its applications in bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept ...
. More regiospecific and less bioorthogonal requirements are best served by the traditional Huisgen cycloaddition, especially given the low yield and synthetic difficulty of synthesizing a strained cyclooctyne
Cyclooctyne is the cycloalkyne with a formula . Its molecule has a ring of 8 carbon atoms, connected by seven single bonds and one triple bond.
Cyclooctyne is the smallest cycloalkyne that is stable enough to be isolated, although the chemical i ...
(compared to the addition of a terminal alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
).
The bioorthogonality of the reaction has allowed the Cu-free click reaction to be applied within cultured cells, live zebrafish
The zebrafish (''Danio rerio'') is a species of freshwater ray-finned fish belonging to the family Danionidae of the order Cypriniformes. Native to South Asia, it is a popular aquarium fish, frequently sold under the trade name zebra danio (an ...
, and mice.
The absence of exogenous metal catalysts makes the Cu-free chemical reactions suitable for the ''in vivo'' applications of bioorthogonal chemistry or bioorthogonal click chemistry.
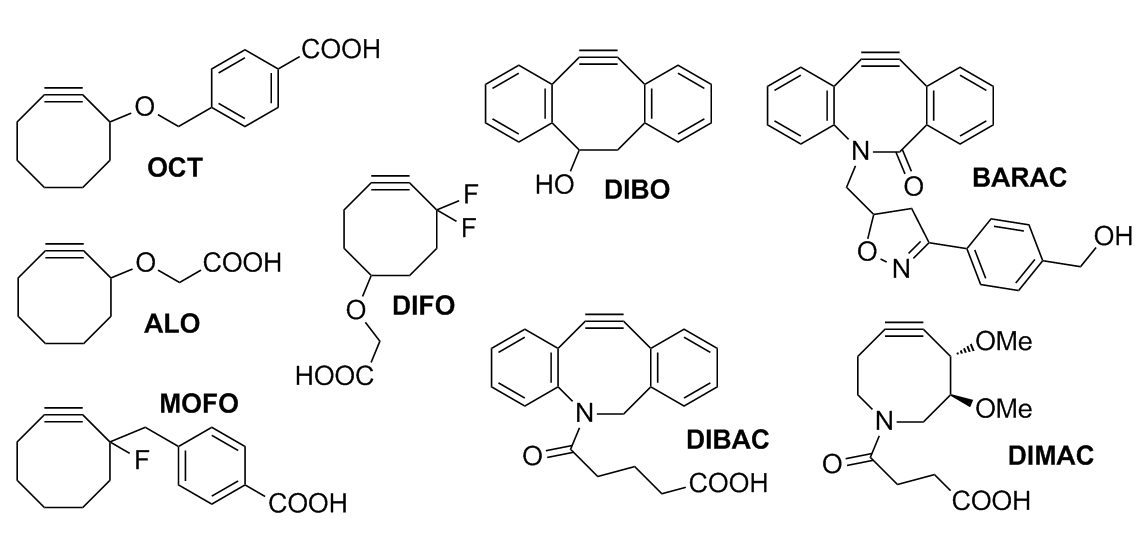
Development of cyclooctynes

Fluorinated cyclooctynes
The cyclooctane derivative OCT was the first one developed for Cu-free click chemistry; it had only ring strain to drive the reaction forward, and the kinetics were barely improved over the Staudinger ligation. After OCT and MOFO (monofluorinated cyclooctyne), the difluorinated cyclooctyne (DIFO) was developed. An improved synthetic approach to a monofluorosubstituted cyclooctyne (MFCO) was introduced that could easily be converted to a useful reactive intermediate for bioconjugation applications, although the reactivity was somewhat slower than DIFO. The MFCO demonstrated excellent stability characteristics for long-term storage. The substituted cyclooctyne is activated for a 1,3-dipolar cycloaddition by itsring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles ar ...
and electron-withdrawing fluorine substituents, which allows the reaction to take place with kinetics comparable to the Cu-catalyzed Huisgen cycloaddition. Ring strain (~18 kcal/mol) arises from the deviation of the bond angles from the ideal 180° to form an eight-membered ring, the smallest of all cycloalkynes. The electron-withdrawing fluorine substituents were chosen due to their synthetic ease and compatibility with living biological systems. Additionally, the group cannot produce cross-reacting Michael acceptors that could act as alkylating agents toward nucleophilic species within cells.
Like most cyclooctynes, DIFO prefers the chair conformation in both the ground state and the minimum energy traction path, although boat transition states may also be involved. Gas phase regioselectivity is calculated to favor 1,5 addition over 1,4 addition by up to 2.9 kcal/mol in activation energy in the gas phase; solvation corrections give the same energy barriers for both regioisomers, explaining the regioisomeric mix that results from DIFO cycloadditions. While the 1,4 isomer is disfavored by its larger dipole moment (all electron-rich substituents on one side), solvation stabilizes it more strongly than the 1,5 isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
, eroding regioselectivity. Experimental studies by Carolyn R. Bertozzi
Carolyn Ruth Bertozzi (born October 10, 1966) is an American chemist and Nobel laureate, known for her wide-ranging work spanning both chemistry and biology. She coined the term " bioorthogonal chemistry" for chemical reactions compatible with ...
report a nearly 1:1 ratio of regioisomers, confirming the predicted lack of regioselectivity in the addition.
Furthermore, nearly all of the distortion energy (92%) arises from the distortion of the 1,3 dipole rather than the cyclooctyne, which has a pre-distorted ground state geometry that increases its reactivity. Fluorination decreases the distortion energy by allowing the transition state to be achieved with a lesser distortion of the 1,3-dipole during a reaction, resulting in a larger dipole angle.
Aryl cyclooctynes
Fusion of a cyclooctyne to two aryl rings increases the reaction rate, and the cyclooctyne reagents of the Bertozzi group proceeded through a series of fusions that sought to increase the ring strain even further. DIBO (dibenzo cyclooctyne) was developed as a precursor to BARAC (biarylazacyclooctynone), although calculations had predicted that a single fused aryl ring would be optimal. Attempts to make a difluoro benzo cyclooctyne (DIFBO) were unsuccessful due to the instability of the compound. The reason for the instability of DIFBO is that it is so reactive that it spontaneously trimerizes to form two asymmetric products that can be characterized by X-ray crystallography. To stabilize the DIFBO, it is trapped by forming a stable inclusion complex with β-cyclodextrin in aqueous media. This complex, formed with the β-cyclodextrin, can then be stored as a lyophilized powder. To obtain the free DIFBO, the lyophilized powder is dissociated with organic solvents to produce the free DIFBO for in situ kinetic and spectroscopic analysis.{{Cite journal , last1=Sletten , first1=Ellen M. , last2=Nakamura , first2=Hitomi , last3=Jewett , first3=John C. , last4=Bertozzi , first4=Carolyn R. , date=2010-08-25 , title=Difluorobenzocyclooctyne: Synthesis, Reactivity, and Stabilization by β-Cyclodextrin , journal=Journal of the American Chemical Society , language=en , volume=132 , issue=33 , pages=11799–11805 , doi=10.1021/ja105005t , issn=0002-7863 , pmc=2923465 , pmid=20666466 Problems with DIFO with in vivo mouse studies illustrate the difficulty of producing bioorthogonal reactions.References