Chromium Hydride on:
[Wikipedia]
[Google]
[Amazon]
Chromium hydrides are compounds of
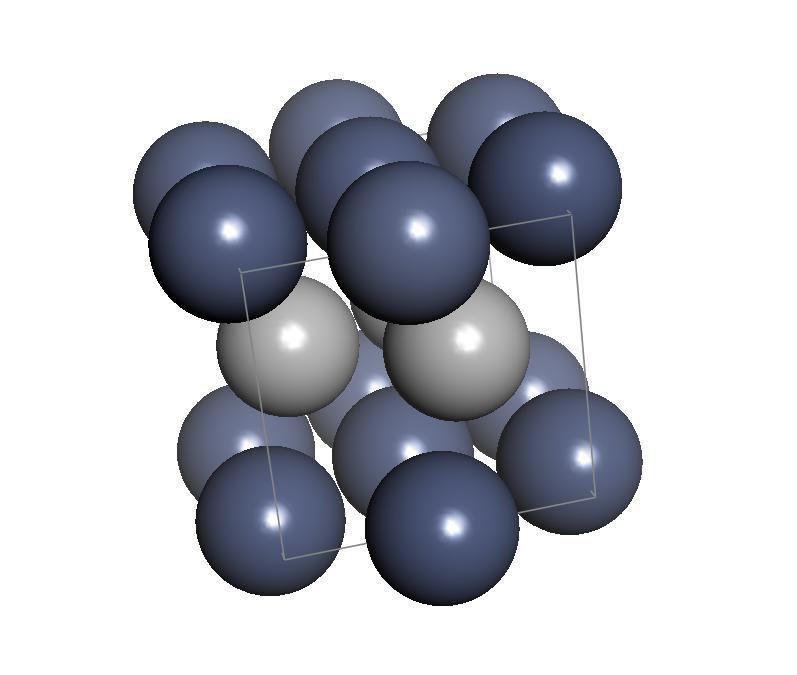
 An apparent unusual allotrope of chromium in a hexagonal crystal form was investigated by Ollard and Bradley by X-ray crystallography; however they failed to notice that it contained hydrogen.
The
An apparent unusual allotrope of chromium in a hexagonal crystal form was investigated by Ollard and Bradley by X-ray crystallography; however they failed to notice that it contained hydrogen.
The  Face-centered cubic CrH had the composition CrH1.7. But in theory it would be CrH2 if the substance was pure and all the tetrahedral sites were occupied by hydrogen atoms. The solid substance CrH2 appears as a dull grey or brown colour. Its surface is easily scratched, but that is due to the brittleness of the hydride.
Face-centered cubic chromium hydride also forms temporarily when chromium metal is etched with
Face-centered cubic CrH had the composition CrH1.7. But in theory it would be CrH2 if the substance was pure and all the tetrahedral sites were occupied by hydrogen atoms. The solid substance CrH2 appears as a dull grey or brown colour. Its surface is easily scratched, but that is due to the brittleness of the hydride.
Face-centered cubic chromium hydride also forms temporarily when chromium metal is etched with
chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, and possibly other elements. Intermetallic compounds with not-quite-stoichometric quantities of hydrogen exist, as well as highly reactive molecules. When present at low concentrations, hydrogen and certain other elements alloyed with chromium act as softening agents that enables the movement of dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to sli ...
s that otherwise not occur in the crystal lattice
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystal, crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that ...
s of chromium atoms.
The hydrogen in typical chromium hydride alloys may contribute only a few hundred parts per million in weight at ambient temperatures. Varying the amount of hydrogen and other alloying elements, and their form in the chromium hydride either as solute elements, or as precipitated phases, expedites the movement of dislocations in chromium, and thus controls qualities such as the hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to plastic deformation, such as an indentation (over an area) or a scratch (linear), induced mechanically either by Pressing (metalworking), pressing or abrasion ...
, ductility
Ductility refers to the ability of a material to sustain significant plastic Deformation (engineering), deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic def ...
, and tensile strength
Ultimate tensile strength (also called UTS, tensile strength, TS, ultimate strength or F_\text in notation) is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials, the ultimate ...
of the resulting chromium hydride.
Material properties
Even in the narrow range of concentrations that make up chromium hydride, mixtures of hydrogen and chromium can form a number of different structures, with very different properties. Understanding such properties is essential to making quality chromium hydride. Atroom temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
, the most stable form of pure chromium is the body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
(BCC) structure α-chromium. It is a fairly hard metal that can dissolve only a small concentration of hydrogen.
It can occur as a dull brown or dark grey solid in two different crystalline forms: face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
with formula CrH~2 or a close packed hexagonal solid with formula CrH~1. Chromium hydride is important in chrome plating
Chrome plating (less commonly chromium plating) is a technique of electroplating a thin layer of chromium onto a metal object. A chrome plated part is called ''chrome'', or is said to have been ''chromed''. The chromium layer can be decorativ ...
, being an intermediate in the formation of the chromium plate.

 An apparent unusual allotrope of chromium in a hexagonal crystal form was investigated by Ollard and Bradley by X-ray crystallography; however they failed to notice that it contained hydrogen.
The
An apparent unusual allotrope of chromium in a hexagonal crystal form was investigated by Ollard and Bradley by X-ray crystallography; however they failed to notice that it contained hydrogen.
The hexagonal close packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occ ...
crystalline substance they discovered actually contains CrHx with x between 0.5 and 1. The lattice for the hexagonal form had unit cell dimensions a=0.271 nm and c=0.441 nm. The crystal form has been described as anti-NiAs
Nias (, Nias: ''Tanö Niha'') is an island located off the western coast of Sumatra, Indonesia. Nias is also the name of the archipelago () of which the island is the centre, but also includes the Batu Islands to the southeast and the small ...
structure and is known as the β-phase. Also known as ε-CrH, the space group is Fmm with hydrogen only in octahedral sites.
A face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
(fcc) phase of chromium hydride can also be produced when chromium is electrodeposited. Cloyd A. Snavely used chromate in sugar syrup cooled to about 5 °C and with a current density of 1290 amperes per square meter. The unit cell dimension in the material was 0.386 nm. The material is brittle and easily decomposed by heat. The composition is CrHx, with x between 1 and 2. For current density above 1800 amps per square meter and at low temperatures, the hexagonal close-packed form was made, but if the current was lower or the temperature was higher, then regular body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
chromium metal was deposited. full text available The condition for preferring the formation of face-centered cubic chromium hydride is a high pH. The fcc form of CrH has hydrogen atoms in octahedral sites in the P63/mmc spacegroup.
 Face-centered cubic CrH had the composition CrH1.7. But in theory it would be CrH2 if the substance was pure and all the tetrahedral sites were occupied by hydrogen atoms. The solid substance CrH2 appears as a dull grey or brown colour. Its surface is easily scratched, but that is due to the brittleness of the hydride.
Face-centered cubic chromium hydride also forms temporarily when chromium metal is etched with
Face-centered cubic CrH had the composition CrH1.7. But in theory it would be CrH2 if the substance was pure and all the tetrahedral sites were occupied by hydrogen atoms. The solid substance CrH2 appears as a dull grey or brown colour. Its surface is easily scratched, but that is due to the brittleness of the hydride.
Face-centered cubic chromium hydride also forms temporarily when chromium metal is etched with hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
.
The hexagonal form spontaneously changes to normal chromium in 40 days, whereas the other form (face-centered cubic) changes to the body-centered cubic form of chromium in 230 days at room temperature. Ollard already noticed that hydrogen is evolved during this transformation, but was not sure that the hydrogen was an essential component of the substance, as electrodeposited chromium usually contained hydrogen. Colin G Fink observed that if the hexagonal form was heated in a flame that the hydrogen would quickly burn off.
Electroplating chromium metal from a chromate solution involves the formation of chromium hydride. If the temperature is high enough the chromium hydride rapidly decomposes as it forms, yielding microcrystalline body-centered cubic chromium. Therefore, to ensure that the hydride decomposes sufficiently rapidly and smoothly, chromium must be plated at a suitably high temperature (roughly 60C to 75C, depending on conditions). As the hydride decomposes, the plated surface cracks. The cracking can be controlled and there may be up to 40 cracks per millimeter. Substances on the plating surface, mostly chromium sesquioxide
Chromium(III) oxide (or chromia) is an inorganic compound with the formula . It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.
Structure and properties
has the corundum s ...
, are sucked into the cracks as they form. The cracks heal over and newer electroplated layers will crack differently. When observed with a microscope the electroplated chromium will appear to be in the form of crystals with 120° and 60° angles, but these are the ghosts of the original hydride crystals; the actual crystals that finally form in the coating are much smaller and consist of body centered cubic chromium.
Superhexagonal chromium hydride has also been produced by exposing chromium films to hydrogen under high pressure and temperature.
In 1926 T. Weichselfelder and B. Thiede claimed to have prepared solid chromium trihydride by reacting hydrogen with chromium chloride Chromium chloride may refer to:
* Chromium(II) chloride, also known as chromous chloride
* Chromium(III) chloride, also known as chromic chloride or chromium trichloride
* Chromium(IV) chloride, unstable
{{Short pages monitor
*
*
*
*
*
*
{{Hydrides by group
Chromium alloys
Metal hydrides